Eye examinations in school children with autism spectrum disorder
A case series to assess strategies and assistive devicesPurpose: To present a case series of how five Autism Spectrum Disorder (ASD) patients completed a comprehensive eye examination when visual, sensory, and communication supports and tests and techniques minimizing tactile defensiveness were employed.
Methods: Patients, between 9 and 17 years, with a confirmed diagnosis of ASD completed an eye examination using a protocol designed to accommodate communication, sensory processing, and motor challenges. Parents provided information regarding verbal communication level (nonverbal, uses short words, verbal).
Results: 5 patients (2 females, 3 males) completed the protocol. Two were verbal and three were nonverbal or minimally verbal. Of these, 1 patient used a communication device to response Yes/No, 1 reviewed a social story in advance of the examination, 2 used a Lea acuity response card, 1 used the palm of a hand in place of an occlude, 1 utilized a sensory fidget.
Conclusion: ASD patients, including those who are nonverbal or limited verbal language as reported by their parents, vary in their needs and responses, but can complete many vision and eye tests in a comprehensive eye examination when supports are provided.
Introduction
Autism Spectrum Disorder (ASD) is a developmental disability that occurs in approximately 1 out of 54 children in the United States.1 ASD is characterized by difficulties in reciprocal social interaction, verbal and non-verbal communication and processing sensory stimuli. 2 These patients often have difficulty responding to subjective vision testing and completing medical procedures. Primary care providers acknowledge decreased confidence in their ability to provide care to these patients. 3 Not surprisingly, ASD patients are less likely to receive the same level of care as typically developing (TD) peers. 4 Parents of ASD children are less confident in the level of care that their children do receive. 5
In vision care, multiple challenges in providing examinations have been identified in the literature. Children with ASD do not always understand the task involved in a vision test or procedure. They may quit responding before the test is complete. Poor responses may be attributed to social and communication deficits, even when they are the result of undiagnosed vision problems. Outcomes of vision examinations vary greatly, according to the testing approach and supports provided.6 Testability reported for visual acuity, ranges from 11 to 100 percent with differences attributed to patients’ age, severity of autism, amount of previous therapy and education. 7-10 Coulter and colleagues reported the results of a vision and eye examination protocol that included visual, sensory, and communication supports finding testability results for ASD children to be comparable to those of typically developing children for all tests, except iCare intraocular pressure tonometry. 10
Case examples enable clinicians to analyze case presentations, separate meaningful information from extraneous data, and connect case to knowledge gained by practice or from the literature.11 As stated by Carey, " The stories of our patients make up the essence of the tradition and culture in medicine." 12 Case examples seed hypotheses for future research, influence expectations for standard of care, educate health care providers by documenting and reporting clinicians' experiences, and provide details of clinical practice. 13-22
Streff, in 1975, reported a case describing optometric care of a four-year old child with characteristics of autism.23 Though Streff’s report is almost fifty years old, today’s eye care providers will appreciate its relevance. Streff noted “autistic children often act as if they are overpowered by visual stimuli while at one and the same time they are in some way ignoring it”. Today, this would be interpreted as avoiding and seeking visual stimuli. Streff also described transitional challenges found in ASD individuals; “We then moved into a testing room. The transition elicited the familiar behavior related to change i.e., crying and screaming”. Over several visits, Streff obtained ophthalmic examination findings, including retinoscopic findings and Hirschberg testing to quantify an esotropia.
Streff’s description of the challenges in examining ASD patients is still true, though our understanding of these challenges and the tools used to address them have advanced.
Since Streff’s time, research on vision findings in the ASD population has emerged. ASD individuals typically show normal visual acuity at distance, but reduced visual acuity at near.24-29 There is an increased frequency of significant refractive error, especially astigmatism. 29-39 ASD individuals are more likely to demonstrate a significant accommodative lag.40 In binocular vision, ASD individuals are more likely to demonstrate reduced convergence and stereoacuity and are at increased risk for strabismus. 25, 29, 36, 38- 42 Saccades and pursuits demonstrate decreased accuracy. 43,44 Differences in ocular health findings are also present. Tonic pupil size is significantly larger. 43 Pupillary response includes a longer latency before responding to light, reduced constriction amplitude and shorter constriction/redilation time than that of typically developing peers.45 Ocular imaging studies have suggested that inner retinal layers thin as they approach the optic nerve head and that optic nerve hypoplasia is more likely.
Individuals with ASD also often demonstrate "lateral gazing”, a behavior in which ASD individuals peer out of the temporal corner of their eye as they turn their head in the opposite direction. Lateral gazing is thought to stimulate the use of peripheral vision to see objects better.43
Supports and Modifications
A variety of supports and modifications are available to aid ASD patients in responding to tests and completing procedures. A social story is a narrative incorporating pictures to describe the steps of an event. For these patients, an eye examination social story was created to depict the sequence of events from arriving at the clinic, meeting the doctor, entering the examination room, and performing tests and procedures. Families could read the social story to the patient before the examination so that the patient knew what to expect and could identify helpful strategies and behaviors.46-51
A support used to help patients to transition from one task to another is a visual schedule. The visual schedule uses a picture sequence to enable ASD individuals to understand what is happening and what they should do; consequently, their ability to respond increases and their anxiety level decreases. 52 The visual schedule was available to the patient and the examiner, in both a laminated card format and within an IPad/Iphone app called First/Then. 53
To increase patients’ comprehensions of test instructions, examiners used instruction sets that were brief, concrete, and specific. For abstract concepts, instructions were paired with pictures, gestures, and visual representations so patients could better understand what they were expected to do. 54
To enable patients to communicate their responses, both low and high technologies were incorporated into the examination.55 For patients who struggled to answer verbally to subjective visual acuity testing, several modifications were incorporated. Patients, who used an augmentative communication device, responded this way to testing. Patients could also respond by pointing or touching their answer on the Lea Symbols Response Panel. When the examiner had questions about what the patient was able to see, communication was achieved using their augmentative device by responding “Yes” or “No”.
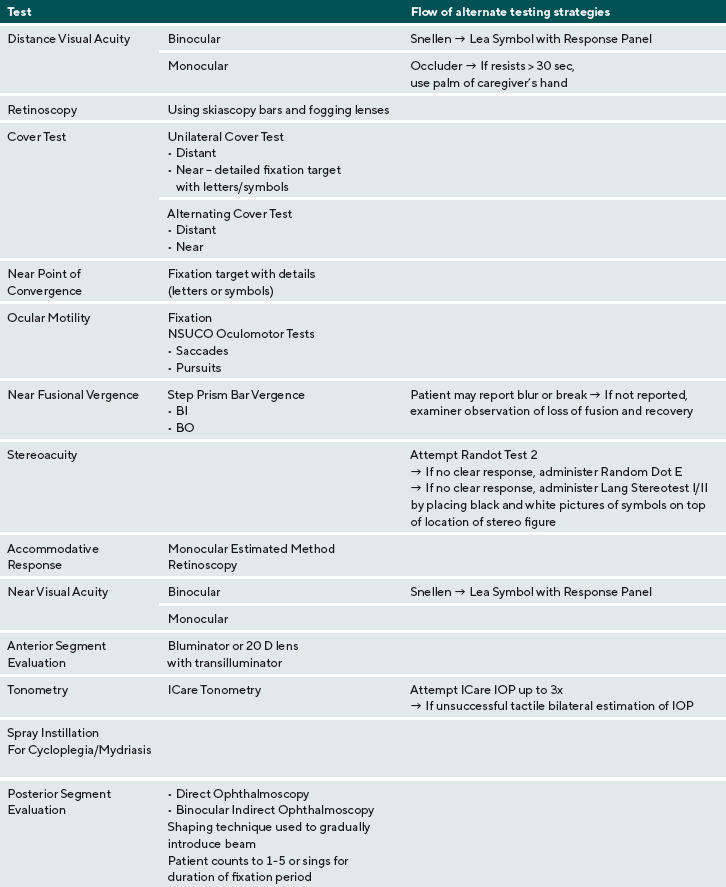
Many ASD patients are hypersensitive to tactile stimuli and resist testing that has a tactile element. This includes wearing polaroid glasses or goggles, holding an occluder over an eye, or placing their face against a head or chin rest. To avoid tactile triggers of sensitivity, non-tactile or minimally tactile tests were used. For example, for patients who would not wear goggles or glasses, the Lang stereotest I was used to measure stereoacuity. For the ocular health examination, a hand-held illuminated magnifier, the Bluminator or a direct ophthalmoscope was used in place of a slit lamp, when necessary to examine the anterior segment. Tonometry readings were obtained using the ICare tonometer. Up to three attempts were made to measure the IOP. If a readable finding was not obtained after three attempts, tonometry testing was discontinued and tactile palpation was used to estimate eye pressure. During tonometry and ophthalmoscopy, a video was played on a portable device to encourage the patient to fixate at the desired point.
To help patients stay on task during procedures that were challenging or required sustained concentration, sensory fidgets and toys were offered. 56, 57
This case series describes how the vision, sensory, and communication supports describe above were used for five patients to complete an eye examination.
Material and Methods
Five ASD children completed a comprehensive eye examination. All data was de-identified to conceal patient’s identity. Parents provided ocular and medical history including the age when diagnosed with ASD, whether the patient wore a refractive correction, had ever had an eye examination, and the patient’s age at the last eye examination. Parents specified the patient’s verbal communication level as nonverbal, minimally verbal/uses short words, or verbal/able to speak fluently. Parents indicated if a patient used an augmentative communication device to communicate. All ASD diagnoses were confirmed by a psychologist with expertise in ASD diagnosis using DSM-IV-TR criteria, after reviewing previous evaluations and the patient’s score on the Social Communication Questionnaire. Prior to the examination, parents were offered a social story about the eye examination. A signed HIPAA authorization was obtained from the patient’s parent for the use and disclosure of their Protected Health Information.
All patients completed a comprehensive eye examination. The following measurements were taken at the eye examination visit (listed in order of administration): binocular distance visual acuity, distance monocular distance visual acuity- OD then OS, retinoscopy, cover/uncover (unilateral cover) test and alternate cover test with prism neutralization at distance and near, near point of convergence, ocular motility testing, near fusional vergence (break and recovery) measured by step prism bar, stereoacuity, Monocular Estimation Method Retinoscopy, binocular near visual acuity, monocular near visual acuity- OD then OS. All testing at near was performed at 40 cm. Testing also included ocular health evaluation: anterior segment evaluation, tonometry measured by ICare tonometry, and posterior segment evaluation. A combination mydriatic-cycloplegic spray of 0.5% tropicamide, 0.5% cyclopentolate, and 2.5% phenylephrine was instilled to both eyes simultaneously to create cycloplegia and mydriasis, thirty minutes prior to posterior segment evaluation.
To encourage the patient to tolerate the bright beam of the binocular indirect ophthalmoscope, the light beam was presented incrementally using a shaping technique was used. The examiner showed light first on the examiner’s hand first and then on the patient’s leg. The examiner then counted for 5 seconds holding the light beam on the patient’s leg and then backed away. This pattern was repeated as the beam was shown the patient’s hand, face, and eye. The examiner then put the BIO on his or her head and shined the beam into the patient’s eye and counted for 5 seconds. A video on a portable device or a lighted toy were held to direct the patient’s direction of gaze during the examination of the peripheral fundus.
Results
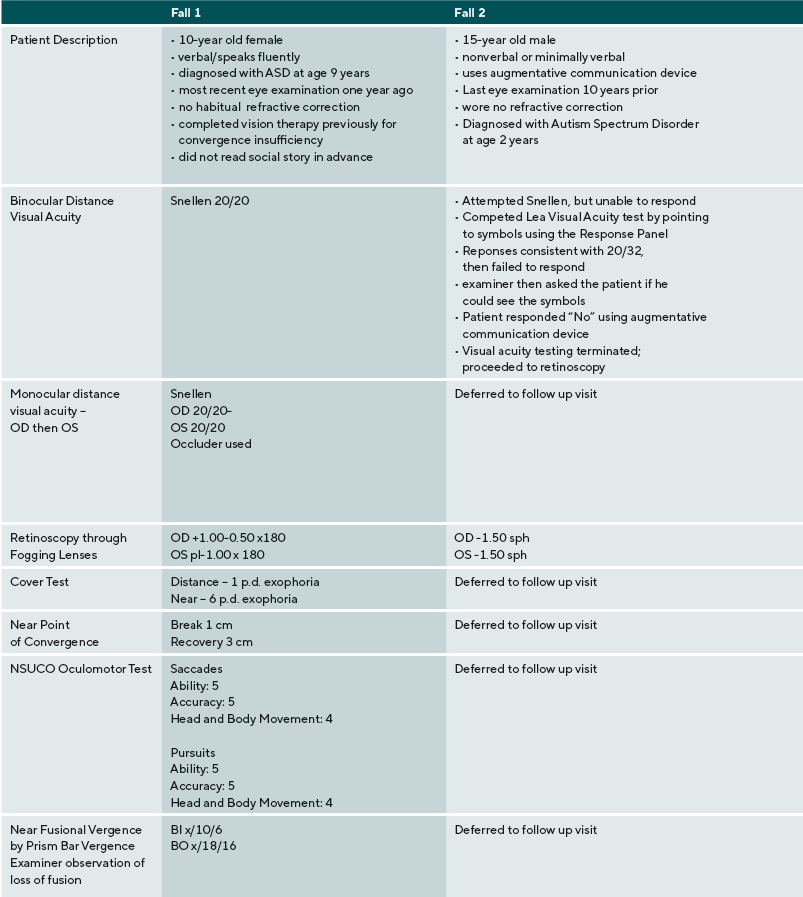
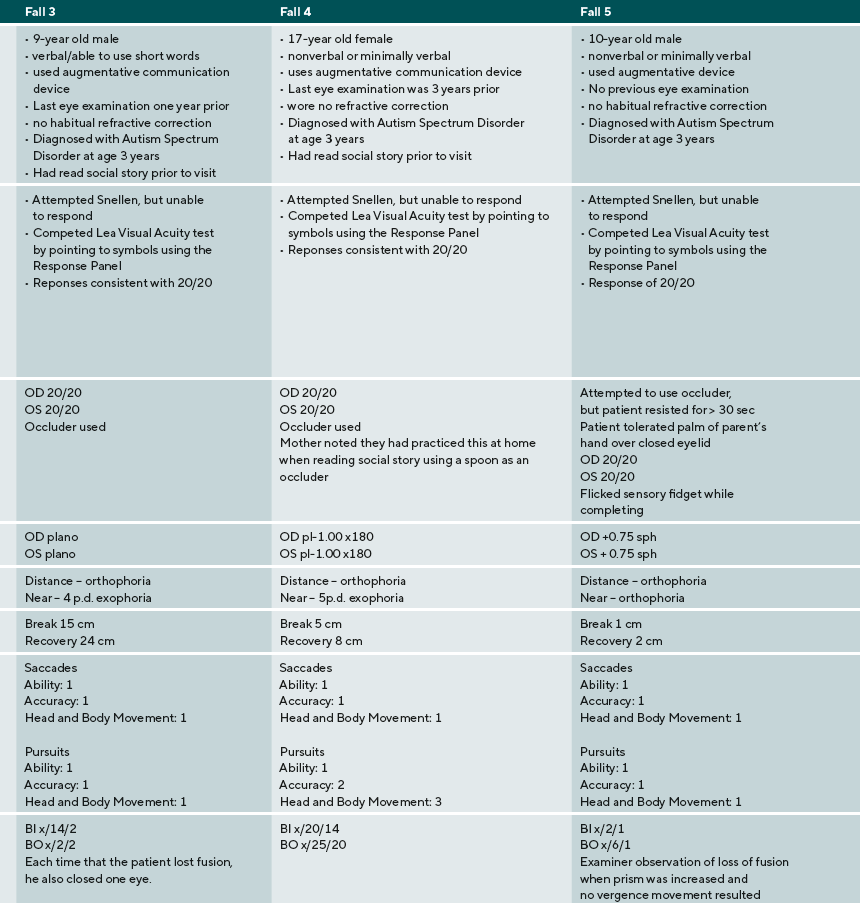
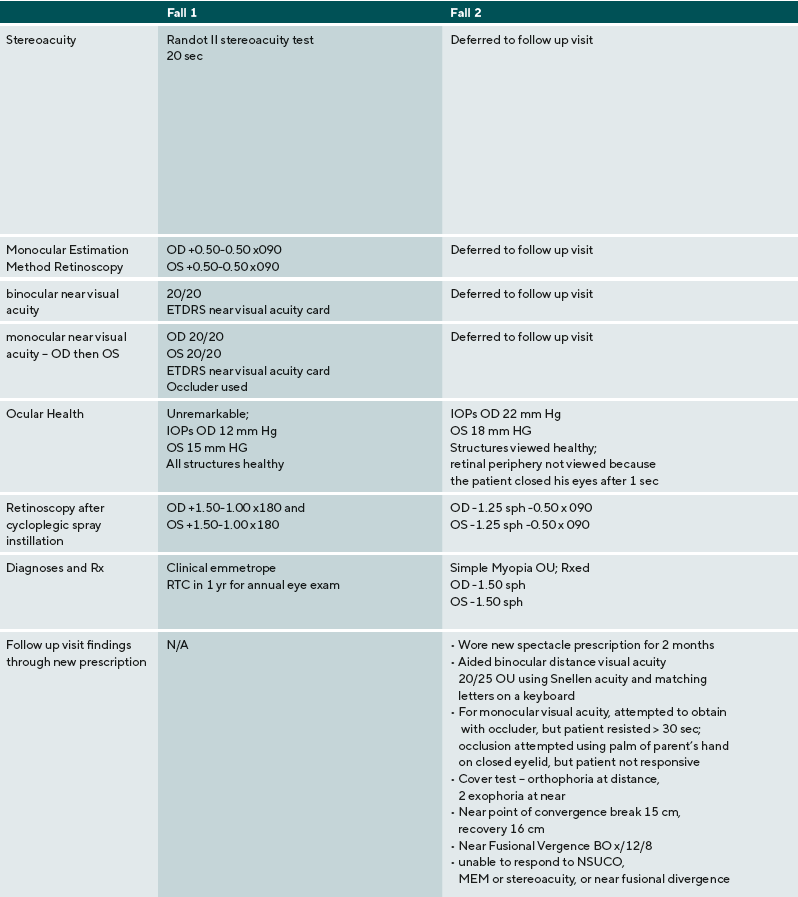
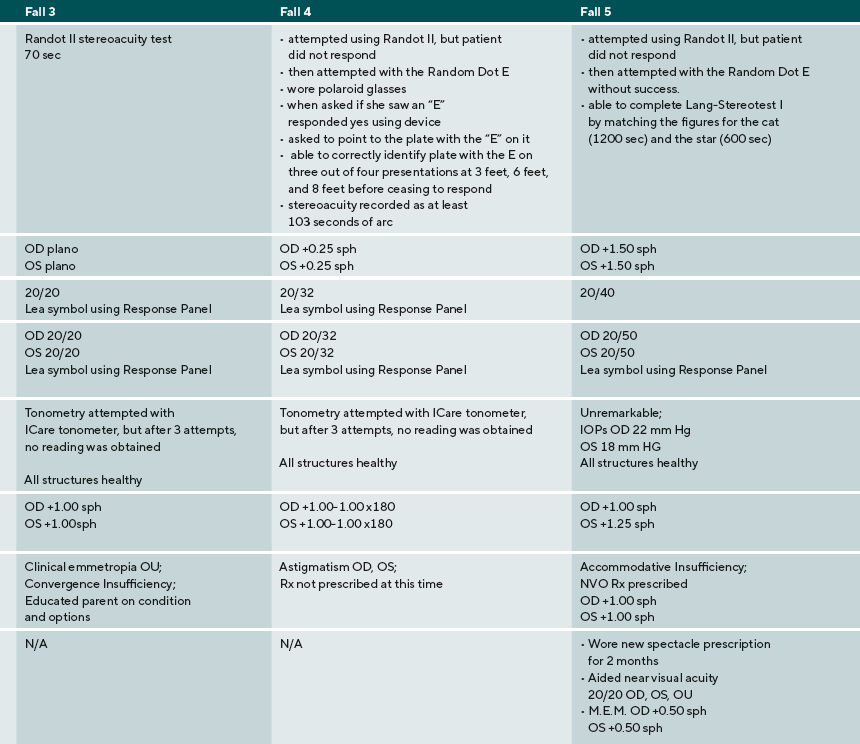
A summary of the eye examination process, supports, and diagnoses are provided for five ASD children: three males, two females. Their ages ranged from 9 to 17 years old. Three were nonverbal or minimally verbal, one was verbal using short words, one was fluent. Findings and detailed responses are presented in Table 2.
Discussion
ASD patients vary greatly in their ability to interact, communicate, and complete vision testing. This case series reflects the wide variety of patient presentation and captures common challenges. as well as possible solutions. Case 1 presents a patient with ASD with a high level of verbal communication whose ability to complete vision test and findings differ little from a typically developing patient. For many of these patients, their ASD is diagnosed relatively late and by school age, they require few supports. Of note, this patient was diagnosed late with ASD, due to the subtle manifestation of the condition.
In contrast, case 2 presents a patient with ASD with a high level of impairment in sensory processing and communication skills. A common dilemma in examining this type of patient is how to gauge when a patient with ASD discontinues a vision test because there is a vision problem versus when they are overwhelmed due to a sensory processing problem, struggling to maintain attention, or cannot meet the cognitive or language demands of the test. Of note, this patient has not had an eye examination for many years and these patients may be particularly at-risk for not obtaining vision care.
The patient begins to respond to visual acuity testing, before the answers given more erratic and are provided more slowly. In this situation, many clinicians might assume that the patient is not high-functioning enough to complete subjective testing. In this case, however, the first expectation is the patient is competent, trying to complete the test, and that this is valid information. By providing a communication opportunity using a YES-NO app and allowing the patient to provide input, the patient responds “no” and the clinician proceeds to determine the visual problem. Retinoscopy, both dry and wet, indicates a refractive error consistent with the patient’s response on distance visual acuity.
The inability to complete a visual acuity test when appropriate supports and tests are used should be viewed with suspicion. In studies of preschool vision screening, the Vision in Preschoolers (VIP) Study Group determined that few children were unable to the Lea Symbols VA test and those who were “unable” were much more likely to have a vision problem.58
For the patient in case 2, due to the detection of a significant amount of refractive error, the examiner defers near point testing until the patient can be corrected and return for testing. To communicate the change in test order, a visual schedule is used. This communicated to the patient what to do and a transitional challenge and behavioral outburst was avoided. Though this patient was not able to complete as many of the vision tests as some other patients, his significant refractive error was corrected.
The parent of the patient in case 3 used the social story prior to the examination, and the patient navigated the testing without any issues. The patient did struggle to respond to distance visual acuity, even though he knew the letters of the alphabet. The patient did not initially recognize what he was supposed to do, however, when a Response Key Card was used to provide a visual support, his performance improved. Case 3 also shows the importance of comprehensive vision care for patients with ASD. Binocular function is important for all patients. Findings including reduced near positive fusional vergence and the near point of convergence are consistent with a diagnosis of convergence insufficiency. Though this patient cannot verbalize enough to describe his symptoms, the observation that he closes an eye each time that he breaks fusion is an important sign consistent with diplopia. Information on the outcomes for treatment of CI in ASD is limited, however, at the very least the parents should be provided information about possible treatment options and that closing an eye is a compensation for a binocular vision problem.
The patient in case 4 also had many challenges in communication, interaction, and sensory processing. Like the patient in case 3, the parent used the social story prior to the examination to familiarize the patient with what she would experience, how she could respond to procedures, and what the examiner would do. Though this patient did not have verbal language, she was able to respond to all vision tests. By using an augmentative device, a smartphone, the patient was able to indicate the letter E and demonstrate that she understood what to do on the Random Dot E test.
The patient in case 5 was very tactile sensitive and initially resisted occlusion to obtain distance monocular visual acuities. He was willing to tolerate his parent’s palm as it was less obtrusive than the occluder. Though this patient did not show significant refractive error, near visual acuities were decreased. Again, this finding could have been interpreted as fatigue at the end of a long examination. When Monocular Estimated Method retinoscopy is performed, however, a significant accommodative lag is detected that is consistent with the patient’s visual acuity at near. For this patient, near correction was prescribed. This case series identifies several clinical points that are important in providing eye examinations to patients with ASD.
Examiners should resist attributing the inability to complete a vision test as secondary to limitations of ASD when using a test that is appropriate to the patient’s behavioral, attention, and communication abilities, without testing visual performance. When a patient fails to complete a test, examiners should consider the possibility that this failure is secondary to a vision problem until findings have indicated otherwise.
Patients with ASD who are nonverbal or have limited abilities to produce speech benefit from comprehensive health and vision care. The quality of care improves by careful observation of patient behavior and the careful review of all test findings including near point tests for accommodative and binocular function. These patients also have considerable demands for clear, single, comfortable vision at near for both educational and therapeutic tasks. To determine if these patients have good visual skills, examiners should assess accommodative and binocular abilities.
Supports and modifications are important and may increase patient’s ability to complete vision testing. This case series has identified several including the social story, visual schedules, visual support (response key card), and augmentative communication through apps for smart phones and tablet computers.
Providing optometrists and other eye care providers with tools to examine patient with ASD is an important step in defining a standard of care for these patients. Skills needed to examine, analyze and diagnose these patients need to be identified so that more practitioners can be trained in examining patients with ASD.59 In addition to technical skills, providers need an attitude that the evaluation of the patient is possible. and the confidence that they can do so. Lindy and colleagues published cross-sectional study of children with autism spectrum disorder aged 6–17 years in the United States or Canada who were enrolled in the Autism Treatment Network Registry.60 Their results indicated that many school-aged children with autism spectrum disorder do not receive recommended vision care.
Conclusions
This case series demonstrates that ASD patients, including those who are nonverbal or have limited verbal language as reported by their parents, can complete many tests in a comprehensive eye examination when supports are provided.
Conflict of interest
The authors have no conflict of interest with regard to the methods and devices mentioned in the article.
J. Pediatr. Health Care, 20, 245-252.
