Literature review on corneal sensitivity with contact lens wear
Purpose: To investigate the effect of contact lens (CL) wear on ocular surface sensitivity and to determine and summarise the relationship between corneal sensitivity and CL comfort.
Material and Methods: A literature search was carried out in PubMed, Scopus and Google Scholar, whereby relevant publications were reviewed and summarised during the period of 13th and 20th November 2023.
Results: For the evaluation of the published literature, it is important to acknowledge that corneal sensitivity measurements are influenced by both, the psychophysical technique and the type of instrument used. Study results show a clear but reversible decrease in corneal sensitivity with oxygen-impermeable polymethyl methacrylate (PMMA) contact lens wear, but little or no effect during daily rigid gas permeable (RGP) CL wear. The results for soft CL wear are more complicated: A decrease in corneal sensitivity may be observed during daily wear of hydrogel CLs with low oxygen permeability, however no change or only a small decrease (depending on the applied measurement method) during silicone hydrogel CL wear. Some studies even found a slight sensitisation with this CL material.
Conclusion: Based on the published study results, it is reasonable to assume that hypoxia is the most likely cause of the reduction in corneal sensitivity during daily CL wear. Successful CL wear with materials that have a sufficiently high oxygen transmissibility has a negligible effect on corneal sensitivity. However, an increased sensation of irritation, particularly with symptomatic CL wear may be observed. This could be caused by (subclinical) inflammatory reactions.
Introduction
Corneal sensitivity is determined by a neurological response of the superficial nerve fibre endings in the corneal epithelium. They register mechanical, chemical and thermal irritation and thus provide the cornea with an important protective mechanism against harmful influences from the environment. They can be functionally differentiated as follows:1,2 Mechanical and electrical stimulation activates the mechanonociceptors, causing a sensation of touch, pain and/or irritation. Polymodal nociceptors react to mechanical, thermal and chemical stimuli, which causes a burning or even stabbing pain. Cold thermoreceptors increase their activity when the temperature on the ocular surface decreases and osmolarity of the tear film increases, which causes a sensation of cooling and dryness. Cold thermoreceptors can be divided into those with a low threshold and high background activity and those with a high threshold and low background activity at normal corneal temperature.1,2 Those with low threshold and high background activity are thought to be responsible for the sensation of cooling, while the others cause the sensation of dryness, irritation and/or pain.3 Cold-sensitive thermoreceptors are involved in the regulation of basal tear film production and blinking.4,5 The sensory nerves change their activity during inflammatory reactions and tissue damage on the anterior surface of the eye,6-9 This causes sensations of irritation and pain and influences the blink frequency and tear film production rate.10,11 An increase in tear film osmolarity stimulates the cold-sensitive and polymodal nociceptors.12,13 An inflammatory event results in a sensitisation of polymodal nociceptors, while cold-thermoreceptors are simultaneously inhibited.6,7 The nerve endings in the cornea and conjunctiva are connected to the lacrimal glands and the orbicularis oculi muscle via a complex feedback network (activating brainstem control circuits) to monitor and maintain the health of the anterior surface of the eye and the tear film at all times.14 They trigger the release of trophic substances (neuropeptides and neurotrophins) to regulate the healing process
after injury.15
Contact lens (CL) wear and the use of care products lead to mechanical forces, temperature fluctuations and chemical stimulation of the ocular surface, either directly through exogenous irritation or indirectly through the release of endogenous agents due to cell damage, hypoxia or changes in pH or osmolarity.16 These stimuli not only lead to stimulation of the sensory nerves, but also to damage to the nerve endings and local inflammation.10 These events in turn further activate and sensitise the sensory nerves, leading to discomfort in some CL wearers.16
Material and methods
A literature search was conducted in PubMed, Scopus and Google Scholar to investigate the influence of CL wear on ocular surface sensitivity. Three searches were conducted using the English keywords ”contact lens AND corneal sensitivity”, ”contact lens AND conjunctival sensitivity” and ”contact lens AND lid margin sensitivity” in the period from 13 - 20 November 2023.
The measurement of corneal sensitivity
The measurement of corneal sensitivity enables the assessment of the functionality of the pain-sensitive superficial corneal nerves. This provides important information about the health of the cornea during the course of a disease process, during the healing phase after an injury or refractive surgery,17-20 as well as during CL wear.16
With the only commercially available instrument, the Cochet-Bonnet (CB) aesthesiometer, a tactile mechanical stimulus is generated with a 6 cm long nylon thread in 0.12 mm and 0.08 mm thickness: depending on the length of this thread, a more or less intense stimulation is triggered on the corneal surface.21 The nylon thread with a thickness of 0.08 mm covers a rather higher sensitivity range, but is unfortunately no longer available. Unfortunately, this instrument has several limitations:22-25 It is an invasive method, which may damage the corneal epithelium; reproducibility is poor because precise centration of the nylon thread is not possible and also the force on the cornea cannot be controlled – even a slight deviation from the correct angle of the thread end to the cornea significantly affects measurement accuracy; the stimulus range is limited, especially in the upper sensitivity range, which means that corneal sensitivity is underestimated and slight sensitivity changes cannot be detected; humidity affects the bending ability of the thread.
To overcome these shortcomings, various prototypes of non-contact air jet aesthesiometers were developed to generate either cooling or warming on the ocular surface, to stimulate temperature-sensitive or mechano- or polymodal nociceptors.26-30 However, it is questioned whether the thermal component of the stimulus can be eliminated to produce a true mechanical stimulus, as the air jet causes an evaporative cooling effect on the cornea depending on the stimulus intensity.31 It is therefore assumed that the mode of action of this type of stimulus results in both a localised reduction in the superficial ocular surface temperature and a slight indentation of the epithelial surface.22,24,32 It is also problematic that the air jet stimulus spreads in a lateral movement over the entire corneal surface, resulting in a stimulus footprint that is difficult to determine.33 It is unclear to what extent the results of previous studies investigating the CL effect on corneal sensitivity were influenced by device limitations and/or differences in the nature of the stimulus.
Recently, a novel non-invasive liquid jet prototype was developed at the University of Applied Sciences, FHNW (Switzerland) (Figures 1 and 2) using small droplets of isotonic saline solution with a pH of 7.4 and an osmolarity of 290.2 mOsm/L adapted to the normal tear film: The Swiss Liquid Jet Aesthesiometer for Corneal Sensitivity (SLACS). The liquid jet emerges from a micro-valve equipped with a heating coil and a temperature sensor. The intensity of the stimulus is controlled with variable pressure levels. In contrast to CB and most other aesthesiometer prototypes, this one uses a software algorithm, which means that measurements can be carried out independently of the influence of the examiner. The functional principle and the relevant physical properties of this new prototype were described,34 and it was clinically validated in a study with 90 participants.35 With its wide stimulus range and pressure resolution, SLACS can potentially detect much smaller variations in sensitivity.
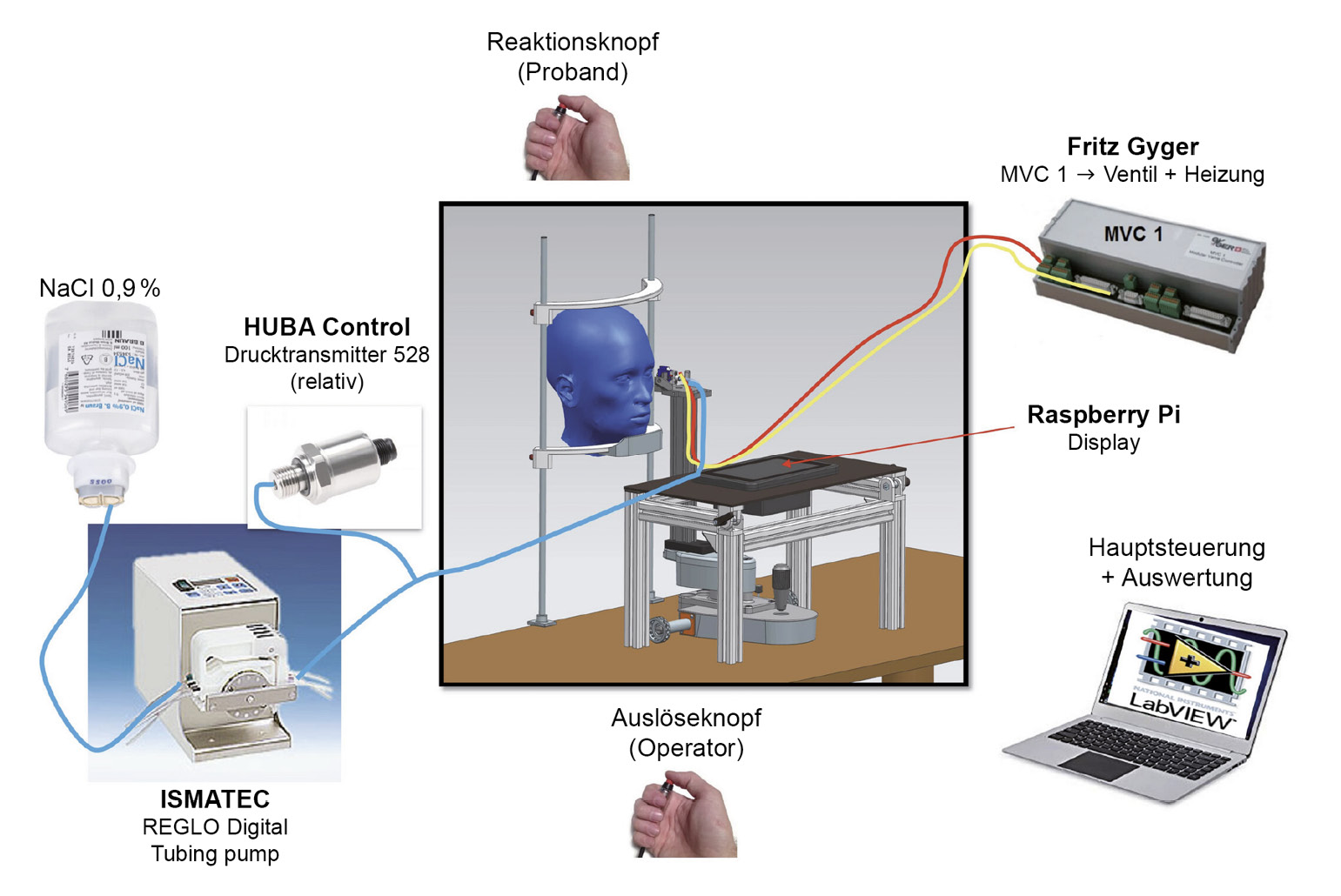

The influence of contact lenses on the sensitivity of the cornea and conjunctiva
CL wear naturally interacts with the ocular surface and can affect corneal sensitivity. It is postulated that the following mechanisms cause a decrease in the sensitivity of the ocular surface when wearing CLs: Metabolic impairment of the cornea due to hypoxia (reduced oxygen supply),36-40 sensory adaptation to mechanical irritation 41,42 and corneal acidosis.43 Metabolic impairment due to hypoxia may be caused by an impairment in the production of the neurotransmitter acetylcholine, which has a higher concentration in the corneal epithelium than in other areas of the body.44 It is therefore assumed that acetylcholine plays an important role in ionic transport (sodium chloride) in the cornea, which in turn has an influence on the generation of nerve impulses.45 A sensory adaptation to mechanical stimulation is plausible due to the altered and reversible arrangement of the nerves in the epithelial subbasal nerve plexus during the orthokeratology CL wear.46 Small changes in the pH value significantly alter nerve activity,43 a reduction in pH occurs as a result of hypercapnia (accumulation of carbon dioxide). Sensitisation of the corneal nerves, on the other hand, is thought to be the result of hyperosmolarity and/or inflammatory mediators during CL wear.27,47 Table 1 summarises all studies and their results that have investigated the influence of CL wear on the sensitivity of the ocular surface.
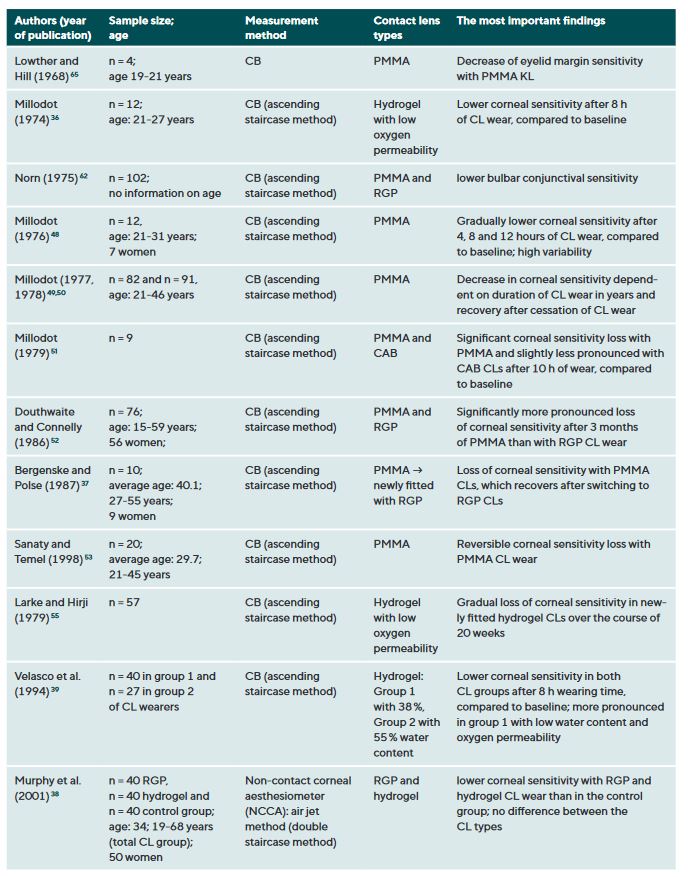
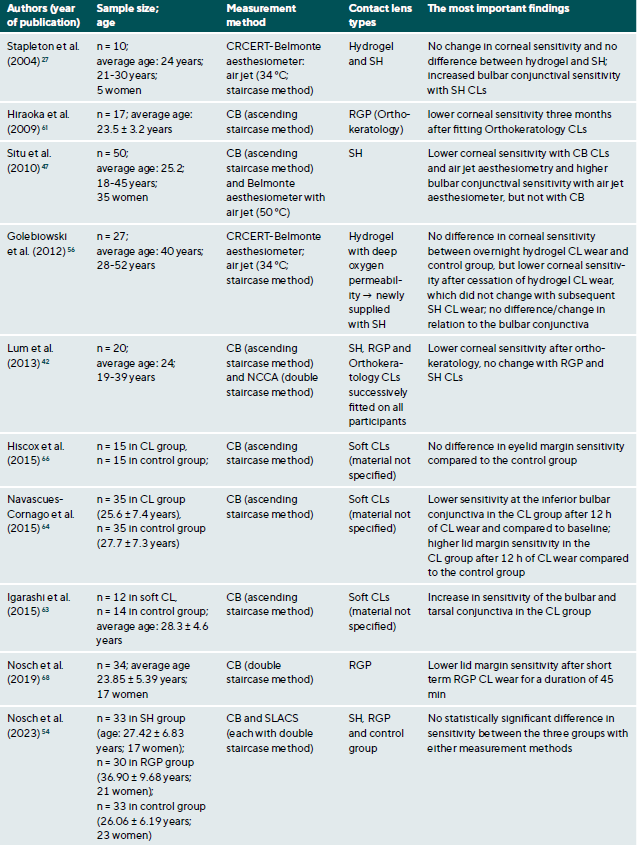
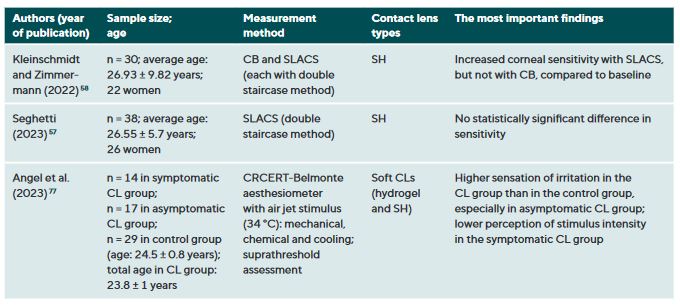
The influence of CL wear on corneal sensitivity
Studies using the CB measurement method observed a clear but reversible decrease in corneal sensitivity when wearing oxygen-impermeable polymethyl methacrylate (PMMA) CLs,37,48-53 but only a slight effect or no effect at all during daily rigid gas permeable (RGP) CL wear.37,38,42,52,54 Even with SLACS, no difference was found between RGP CL wearers and silicone hydrogel (SH) CL wearers and a control group.54
The results to date for soft CL wear are more complicated: the studies conducted with CB agree that corneal sensitivity decreases with hydrogel CLs with a low Dk value (= low oxygen permeability),36,39,55 but not or only to a small extent with SH-CLs with a high Dk value (= high oxygen permeability).42,47 Studies with air jet aesthesiometry and SLACS observed no or only very minor effects in hydrogel and SH-CL wearers with a low or high Dk value.27,42,47,54,56,57 Interestingly, however, in a study with SLACS (but not with CB), a slight sensitisation of the cornea was observed in SH-CL with overnight wear after one week compared to baseline.58 This increased nerve activity could be an expression of a subclinical inflammatory reaction or indicate a certain biochemical stress.
Previously published studies and a literature review by Stapleton et al.59 support the hypothesis that hypoxia is primarily responsible for a decrease in corneal sensitivity when wearing oxygen-impermeable PMMA CLs rather than sensory adaptation to mechanical stimuli: Corneal sensitivity recovered when switching from PMMA to RGP CLs.37 When wearing RGP CLs, only a very slight 52 or no decrease in sensitivity 42,54 was observed compared to a control group 37,42 or a soft CL group.
A hypoxic aetiology is confirmed by the decrease in sensitivity overnight without CL wear 60,61 and the reduced corneal sensitivity a closed eyelid.44
With regard to daily RGP CL wear, the results are promising, as a reduction in corneal sensitivity due to a delayed and/or reduced response of the superficial nerve endings could potentially lead to an increased risk of infection. The short-term and reversible reduction in corneal sensitivity with orthokeratology is likely to be due to the mechanically altered arrangement of nerve fibres in the epithelial subbasal nerve plexus rather than hypoxia.42,46,62
The influence of CL wear on the bulbar conjunctiva
Only few studies looked at the influence of CL wear on the sensitivity of the bulbar conjunctiva and it is therefore unclear whether changes in sensitivity are to be expected: A study conducted with CB observed lower sensitivity with PMMA and RGP CL wear.63 Using air-jet aesthesiometry, two studies found a sensitisation of the bulbar conjunctiva when wearing SH-CLs,27,47 using CB, one study found no change when wearing soft CLs,47 another, however, observed sensitisation.64 In contrast, a lower sensitivity of the inferior bulbar conjunctiva was noted after 12 months of wearing soft CLs compared to a control group and to baseline.65 In contrast, another study found no difference in long-term overnight hydrogel CL wearers with air-jet aesthesiometry compared to a control group.56
The influence of CL wear on the tarsal conjunctiva
According to a study from the 1960s with a very small sample of four participants, the sensitivity of the lid margin (measured with CB) decreases when wearing PMMA, RGP and hydrogel CLs with a low Dk value.66 In contrast, a more recent study observed no change in lid margin sensitivity when wearing modern soft lenses (material not specified),67 another reported a higher lid margin sensitivity compared to a control group.64 Another study also found a sensitisation in the CL group after 12 h of wearing modern soft CLs (materials not specified) compared to the control group.65 This could be caused by a lack of wearing comfort due to interaction between the lid margin and the CL surface. In contrast, another study confirmed a lower lid margin sensitivity after wearing RGP CLs once for 45 min.68 In the search for an answer to the question of which individuals are likely to have fewer adaptation problems with RGP CLs, it also investigated whether spontaneous wearing comfort could correlate with eyelid margin sensitivity at baseline or with the decrease in sensitivity during RGP CL wear. Unfortunately, no correlation could be established. Sensitivity measurements on the eyelid are challenging because they require ectropionisation, which is an unnatural situation for the eye.
When assessing the results of all these studies, it must be borne in mind that the measurement of corneal sensitivity is influenced by both the psychophysical technique and the type of instrument used. Unfortunately, as already mentioned, the only commercially available instrument, the tactile CB aesthesiometer, is unsuitable for everyday clinical use.
Various prototypes have been developed with air jet stimuli to generate either cooling or warming of the ocular surface that stimulate temperature- or mechanically-sensitive or polymodal nociceptors.26-30 However, it is controversial whether the thermal component of the stimulus can be eliminated to produce a true mechanical stimulus, as the air jet produces an evaporative cooling effect on the moist cornea depending on the airflow intensity.31 It is therefore assumed that the mode of action of this type of stimulus causes both local cooling and indentation of the epithelial surface.22,24,32 Another problem is that the air stimulus spreads in a lateral movement over the entire ocular surface, resulting in a stimulus footprint that is difficult to determine.33 The SLACS prototype was developed to overcome this challenge using a small liquid jet that can be adjusted to the temperature of the ocular surface. Unlike CB and most other aesthesiometer prototypes, it uses a software algorithm that is independent of the examiner.
The influence of CL care products
According to studies, care products preserved with polyhexanide in combination with certain SH-CL materials can cause corneal and conjunctival staining as well as bulbar hyperaemia and CL discomfort.69-72 In a crossover pilot study with the CB aesthesiometer, Epstein found reduced corneal sensitivity with polyhexanide-preserved care products compared to polyquad-preserved care products.73 However, these differences were not statistically significant, possibly due to the small sample size. Situ et al. also investigated the effects of different SH CL/care product combinations on the sensitivity of the ocular surface in a larger group of 48 subjects using CB and the air jet anaesthesiometer:47 They compared the sensitivity changes in CL wearers using hydrogen peroxide-based care products and two different multipurpose solutions (preserved with polyhexanide and polyquad/Aldox). They obtained reduced corneal and conjunctival thresholds for chemical sensitivity with the polyhexanide-preserved care product compared to the one preserved with polyquad/Aldox. However, this difference was only statistically significant for the corneal measurements. No statistical differences were found with regard to the tactile and pneumatic mechanical threshold values. They attributed the increased chemical sensitivity using the polyhexanide-preserved care product to the increased prevalence of corneal and conjunctival staining, which could have resulted in activation of the polymodal nociceptors.
What role do the superficial corneal nerves play in CL comfort?
When wearing CLs, a complex and multifactorial stimulation of the functionally different nerve endings is triggered. Impaired comfort is caused by mechanical, altered osmolarity, cooling and/or chemical effects.16
Due to their direct interaction with the front surface of the eye, CLs may cause mechanical irritation. Friction with the ocular is caused by the edge properties of the CLs (rounded or sharp-edged), the rigidity and the surface properties. The tear film, already thinned by the presence of the CL, continues to decrease in thickness, blink frequency increases, resulting in eyelid wiper epitheliopathy 74 and, due to the increased shear forces during a blink, lid-parallel conunctival folds may also form.75 In this situation, the polymodal nociceptors and the mechanoreceptors are stimulated, which leads to a sensation of irritation and foreign body sensation. Inadequate sensory adaptation to CLS also causes discomfort.76 Another consequence of these processes is hyperosmolarity, which stimulates the polymodal and cold-sensitive nociceptors, causing a sensation of dryness, burning and cooling. In this context, inflammatory mediators are also released, which in turn sensitise polymodal nerve endings in the cornea and conjunctiva, which also results in irritation and burning.
The difference between symptomatic and asymptomatic CL weares has been little researched to date: One study showed that symptomatic CL wearers reacted more sensitively to suprathreshold stimuli.76 According to another study, corneal sensitivity decreases with increasing symptoms.77 A recently published study compared the subjective perception of intensity and irritation to suprathreshold mechanical, chemical and cooling stimuli in an asymptomatic, symptomatic CL- and control group using an air jet aesthesiometer.78 They observed a higher sensation of irritation in the CL- than in the control group, especially in asymptomatic CL wearers. In contrast, they recorded a lower intensity sensation to cooling irritation in the symptomatic CL group. They postulate that sensitisation of nociceptors causes irritation on an inflamed and poorly wetting cornea. At the same time, the cold-sensitive nociceptors would reduce their activity due to the inflammatory reaction. In view of the rather small sample with different group sizes, however, these results must be confirmed in a larger-scale study.
In summary, based on studies published to date, hypoxia is the most likely cause of a mechanical reduction in corneal sensitivity during daytime CL wear. Successful daily wear of modern, sufficiently oxygen-permeable CLs has a negligible effect on corneal sensitivity. However, there may be an increased sensation of irritation, particularly with symptomatic CL wear. It is reasonable to assume that (subclinical) inflammatory reactions play a significant role. Further studies are needed to investigate the role of the bulbar conjunctiva and the lid margin (especially in the region of the lid wiper) in comfort problems with modern CL materials, as this is where most interaction with the CL occurs.
COE Multiple Choice Questionnaire
The publication "Literature review on corneal sensitivity with contact lens wear" has been approved as a COE continuing education article by the German Quality Association for Optometric Services (GOL). The deadline to answer the questions is 1st April 2025. Only one answer per question is correct. Successful completion requires answering four of the six questions.
You can take the continuing education exam while logged in.
Users who are not yet logged in can register for ocl-online free of charge here.
in corneal sensitivity with hydrogel contact lenses. Acta Ophthalmol. (Copenh)., 72, 53–56.
corneal sensitivity. Acta Ophthalmol. (Copenh)., 58, 434–439.
corneal health. Optom. Vis. Sci., 6, 699–702.
pneumatic mechanical, and chemical stimulation. Invest. Ophthalmol. Vis. Sci., 51, 6111–6117.
