New Developments in Corneal Cross-Linking for Keratoconus – Enhancing the Shape of the Cornea
Purpose: Corneal cross-linking (CXL) is an established treatment for corneal ectasia, particularly keratoconus. The goal of the treatment is to stabilize the biomechanically weakened cornea, thereby halting further progression of the disease and preventing loss of visual acuity.
Material and Methods: CXL is based on a photo-oxidative process induced by riboflavin (vitamin B2) and ultraviolet A radiation (UVA, 365 nm), which causes the formation of covalent bonds between collagen molecules.
Results: The process depends on four main factors: riboflavin, UVA light, oxygen, and saturation of cross-linking. CXL has been shown to be an effective and safe treatment, which has led to a reduction in the need for corneal transplants for this condition in the past. The standard protocol does not primarily lead to an improvement in visual performance or refractive error because the reduction in higher order aberrations does not seem to be high enough. Thus, the combination with excimer laser treatments is a new approach to treat visual complaints with sufficient clinical results where an improvement in visual acuity could be measured. Otherwise, the treatment can be customized with special irradiation patterns of the UVA light, resulting in a more pronounced flattening and therefore a stronger regularization of the corneal curvature.
Conclusion: The primary purpose of these modifications is not to provide spectacle-free or contact lens-free vision, but to provide better vision with glasses or soft contact lenses, particularly in cases where rigid gas permeable contact lenses are not tolerated
Introduction
Corneal cross-linking (CXL) is an established method to treat keratoconus (KC), thereby halting the progression of the disease. The method was developed by Prof. Seiler and Prof. Spoerl and aimed to stabilize the corneal tissue regarding an increase in biomechanical properties, e.g. stiffness. In the early stage of the development process different methods were investigated to increase the stiffness of the cornea such as photo-sensitizer and ultra-violet light type A (UVA), chemical solutions (e. g. glutaraldehyde), and aldehyde sugars (e. g. glucose).1 In these experiments, the technique using a photosensitizer (riboflavin) and UVA light was found to be most suitable for clinical application because it provided a sufficient increase in corneal stiffness, a localized treatment effect, a short treatment time, and maintenance of the corneal transparency.1,2 In the following years, it was shown that the increasing effect in biomechanical properties depends on four main factors: 1) riboflavin, 2) UVA light, 3) oxygen, and 4) saturation of the cross-linking.3
Corneal ectasia is characterized by a steepening in corneal curvature, thinning of corneal thickness, increasing myopia as well as irregular astigmatism leading to a loss of vision. The most common clinical manifestation are KC and pellucid marginal degeneration (PMD). KC in particular is a disease that often develops at a younger age, where it has an obvious negative impact on the quality of life.4,5 Due to the progressive nature of the disease, it was the reason for the majority of corneal transplantations until the 2000s.6 The bulging of the cornea is attributed to the fact that the properties of the tissue are biomechanically weakened in terms of a reduced elastic modulus.7,8 The cycle of biomechanical decompensation in corneal ectasia is assumed to be as follows: First, there is a focally reduced elastic modulus within the cornea. This leads to the cornea exhibiting an asymmetric distribution of biomechanical properties, which is followed by a thinning pro cess of the tissue. This in turn leads to an increase of stress in this area and therefore causes a deformation (increased curvature) of the cornea.9 However, it is unknown what the exact reason for the biomechanical weakening of the cornea is. The structure of collagen, the composition of proteoglycans and their binding to collagen fibers determine the biomechanical properties of the cornea. These factors have been shown to be altered in KC.10,11,12 As a consequence, cross-linking within the collagen and/or between individual collagen molecules must be impaired.
CXL compensates for the structural disorder in corneal ectasia by forming new covalent bonds between collagen fibers, increasing the strength of the cornea to restore its adequate biomechanical stability.
Wollensak et al. published the first clinical results demonstrating that riboflavin and UVA light based cross-linking stop the further progression of KC with little side effects or complications.2 In 2016, the treatment was approved by the Food and Drug Administration (FDA) in the USA for KC 13 and iatrogenic keratectasia after refractive surgery.14 Furthermore, the establishment of CXL as standard treatment in KC led to a reduction in rates of corneal transplantation.15 The relatively simple procedure and the significantly lower costs result in a highly lucrative treatment in comparison to corneal transplantation, which has significant positive economic impact on national healthcare systems.16,17
Clinical indication of CXL in keratoconus
In Germany, the indication for CXL is progressive KC which was defined by the Federal Joint Committee (G-BA) delegated by the German Government. The definition of progression is stated as an increase in maximum keratometry value (Kmax) ≥ 1 D, an increase in astigmatism in subjective refraction of ≥ 1 D, or decrease of the base curve of the best fitting contact lens of ≥ 0.1 mm. If any of the three criteria are met, then there is an indication for CXL.
Standard CXL protocol
The standard CXL protocol (S-CXL (3*30)) also known as Dresden protocol was proposed using UVA irradiation of 3 mW/cm² for 30 min.2 The procedure is usually performed in a sterile operation room, however, recent studies by Hafeziet al. have shown that it can also be performed at a slit lamp in a sitting position in the outpatient clinic.18 They showed there was no increased risk of complications.19 The corneal epithelium has to be removed (“epi-off” treatment) in local anesthesia before 0.1% riboflavin is applied to the cornea. After riboflavin has saturated the corneal stroma, a corneal thickness of at least 400 μm must be present in order to carry out the treatment according to the standard protocol. Otherwise, an adapted fluence must be applied to the cornea.20 Afterwards, the cornea is irradiated with UVA light. Postoperatively, a soft therapeutic contact lens is applied until the re-epithelialization process is complete. After corneal re-epithelialization, topical steroid treatment is given for 4 weeks.
There are many studies that have shown the safety and efficacy of the treatment in KC eyes with a long-term follow-up.21,22,23 All these studies found a statistically significant improvement in topographic and aberrometric measures as well as in best-corrected visual acuity (BCVA), indicating a stability of the disease. A recent study by the Dresden group demonstrated the long-lasting treatment effect of 15 years with a small rate of re-treatments (8 %) or the necessity of corneal transplantations.24 It could also be confirmed that the improvement in topographic metrics was statistically significant to baseline after 10 and 15 years, however, no further improvement occurred during the 10-, and 15-year period.24 The strongest evidence of treatment success comes from randomized controlled trials conducted in the past in Australia, the United States, and the United Kingdom.13,15,26 These studies reported a reduction of Kmax by −1.0 to −1.6 D in the CXL group, whereas the control group (untreated KC patients) increased by an average of +1.0 to +1.75 D. Also, visual acuity improved compared to untreated eyes. During the study period, a progression of the disease was observed in up to 7 % of treated KC patients, while the untreated KC patients had progression rates between 14 % and 52 %, indicating high efficacy and safety of the CXL treatment.13,25,26
Accelerated protocols versus standard protocol
For clinical practice, the major disadvantage of the Dresden protocol is the long treatment time of 1 hour, which limits the patient’s comfort and the surgeon’s workflow. For this reason, a shorter treatment is desirable. To achieve this goal, the photochemical law of reciprocity (Bunsen-Roscoe law) was employed by decreasing the illumination time and increasing the intensity of the UVA light, whereby the total energy of 5.4 J was not exceeded. Different accelerated protocols (A-CXL) were evaluated in experimental studies on porcine eyes using stress-strain extensometry, which confirmed an equivalent stiffening effect of shorter CXL protocols such as 9 mW/cm² for 10 min and 15 mW/cm² for 6 min in comparison to the S-CXL (3*30).27 Another study showed that the law of Bunsen-Roscoe reciprocity was valid up to 40 to 50 mW/cm² with very short treatment durations of up to 2 min.28 In contrast, Hafezi and co-workers showed that the Young‘s modulus was significantly lower at 10 % strain for A-CXL (9*10) as well as A-CXL (18*5) compared to the S-CXL (3*30).29 They concluded that the treatment efficiency was reduced due to the intrastromal oxygen diffusion capacity and increased oxygen consumption associated with higher irradiances. In another study using inflation tests and air-puff tonometry, the treatment efficacy of the S-CXL (3*30) was more pronounced compared to A-CXL protocols, however, the A-CXL (9*10) was not inferior concerning the stiffening increase compared to control eyes.30 Overall, the experimental results show that the A-CXL with 9 mW/cm² for 10 min leads to an adequate corneal stiffening effect, comparable to the S-CXL protocol.
From a clinical point of view, a similar outcome for S-CXL and A-CXL (9*10) concerning BCVA, keratometry, and corneal thickness was found after 12 months in most of the prospective (randomized) trials.31,32 However, there were indications that S-CXL (3*30) generated more corneal thinning. Several meta-analyses that have been conducted, summarize these comparisons between standard and accelerated cross-linking. Wen et al. analyzed 11 trials showing a greater effect in terms of reduction of Kmax of the S-CXL than A-CXL protocol, while A-CXL induced less reduction in central corneal thickness (CCT) and endothelial cell density (ECD) than S-CXL.33 Another analysis included 22 studies with 1158 eyes, where A-CXL rendered less corneal thinning and S-CXL a deeper demarcation line and greater changes in minimum keratometric values. The authors concluded that overall, S-CXL as well as A-CXL, provide successful results in the strengthening of corneal tissue.34 Considering only randomized trials, it was found that A-CXL showed a comparable efficacy and safety profile at the 12-month follow-up but had less impact on improving best spectacle-corrected visual acuity when compared with the S-CXL protocol. Overall, both methods stopped the disease progression to a similar extent.35 The Dresden group investigated the need for re-treatments (failure rates) after S-CXL and A-CXL in a large dataset.36 120 and 110 patients were included, who received S-CXL or A-CXL, respectively. The protocols were similar, except for the intensity and treatment time. The cumulative survival rate (Kaplan-Meier-Analysis) was 92.5 % and 86.4 % after 3 years, which was not statistically significant. The risk factors for re-treatments were a thinner preoperative thinnest corneal thickness, a higher preoperative Kmax, and the presence of neurodermatitis combined with other atopic diseases. The used protocol (S-CXL or A-CXL) did not have a statistically significant impact on progression after initial CXL. Therefore, the A-CXL (9*10) protocol has been proven to be an effective treatment for progressive KC, leading to a halt of progression and clinically equivalent outcomes.
The combination of CXL with excimer laser treatments
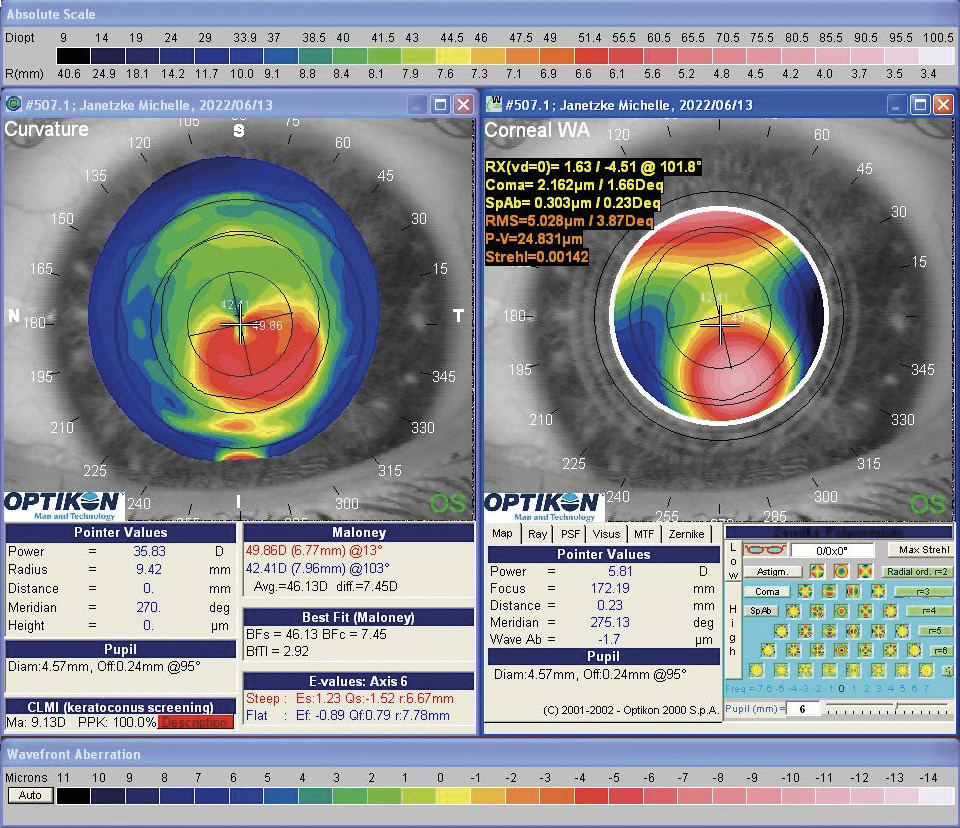
Despite improvements in visual acuity reported in previous studies, individual improvement in visual performance cannot be guaranteed. It should be noted that CXL treatment has only a small effect on uncorrected and spectacle visual acuity. It has been shown that CXL leads to a flattening effect of the anterior corneal curvature of around 1.0 D in most studies. A partial reduction of high-order aberrations could also be observed,37 however, with less impact on visual acuity.37,38 As a result, rehabilitation of visual performance is still only possible via contact lenses or through further surgical interventions (e. g. intrastromal rings/ring segments). When fitting rigid gas permeable contact lenses (RGP) or scleral lenses visual performance can be raised to a satisfactory level compared to uncorrected visual acuity as well as to spectacle visual acuity.39 An important prerequisite for wearing RGP lenses, however, is patient compliance, which is not always given. The compliance problems but also the disease KC itself can furthermore affect the psyche of the patients.40 To address the vision problems of KC patients, CXL can be combined with refractive procedures such as transepithelial photorefractive keratectomy (transPRK). The simultaneous use of both methods, initially the transPRK followed by the CXL, has advantages with regard to the lower burden on the patient and a lower risk of infection due to multiple operations. Such a procedure was first described by Kanellopoulos and Binder (the Athens protocol).41 Recently, they reported their 10-year follow-up data of 144 eyes, in which an improvement in uncorrected and corrected decimal visual acuity from 0.19 ± 0.17 to 0.55 ± 0.19 and from 0.59 ± 0.21 to 0.81 ± 0.19, respectively, could be demonstrated.42 In addition, steepest and maximal keratometry value decreased from 50.57 ± 2.80 D to 44.00 ± 3.22 D as well as from 53.43 ± 2.97 D to 44.75 ± 2.14 D, respectively.42 This indicates a strong regularization of the corneal curvature after the procedure, which also reduces the amount of high-order aberrations. Despite the safety and efficacy of the protocol (stabilization of the ectasia in 94.4 % of cases), the corneal thickness was reduced by around 73 μm, leading to a potential negative effect on the stability of the cornea.42 Alessio et al. were able to show that in a direct comparison between CXL as a standalone treatment and the combination of CXL and PRK, the uncorrected visual acuity in the CXL+PRK group improved significantly compared to the CXL standalone group: Keratometry metrics (steep, flat and maximum keratometry) and the inferior-superior index decreased significantly. As a result, the high-order aberrations and especially coma-like aberrations in the CXL+PRK group were significantly reduced compared to the CXL standalone process.43 In addition, the efficacy and safety of CXL and PRK combined procedures could be demonstrated in other studies.44,45,46 However, the previously described studies did not consider the altered epithelium thickness in KC eyes because it is usually thinned over the cone.47 An example is given in figure 1.
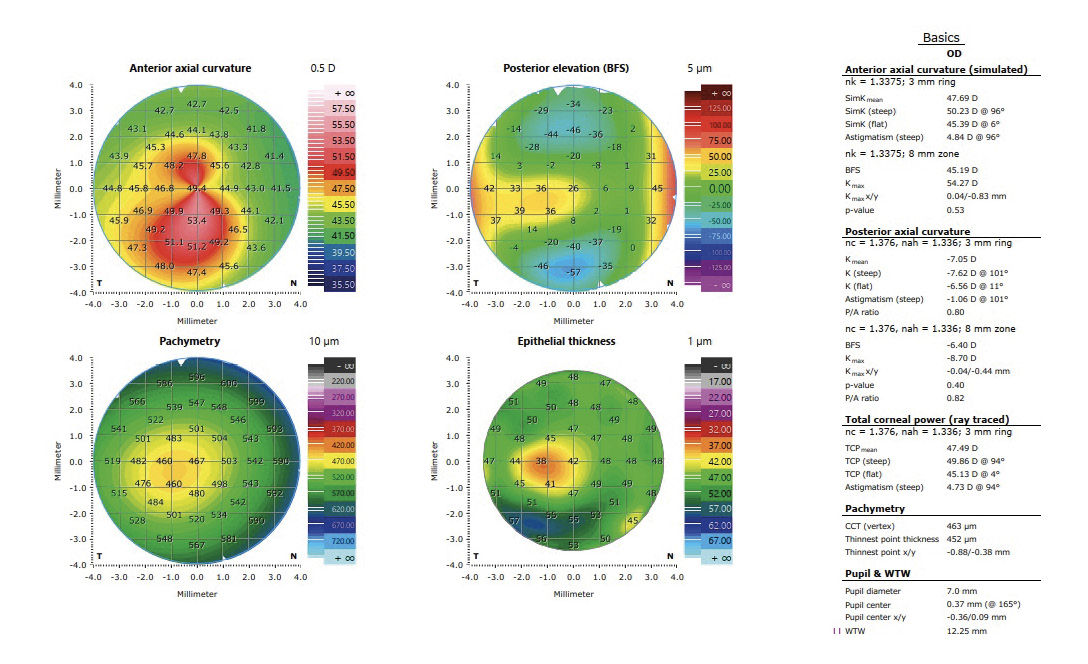
In the treatment planning of the transPRK (Schwind Amaris 750 S, Schwind eye-tech-solutions GmbH, Kleinostheim, Germany) using the Schwind Cam software (Schwind ye-tech-solutions GmbH, Kleinostheim, Germany), an epithelium thickness is reallocated with a central thickness of 55 μm. However, the actual epithelium thickness is usually lower. This fact can be considered in the treatment planning, in which the values are corrected by real measured data from optical coherence tomography (with epithelium mapping). The ablation profile is then recalculated and an unnecessary ablation of stromal tissue can be prevented. Moreover, the Schwind Cam software enables calculations providing a minimal ablation of the tissue that can include only high-order aberrations of both manifest refraction and high-order aberrations. To avoid further biomechanical weakening of the corneal tissue, the maximum ablation of 50 μm of the stromal tissue in the area of the cone should not be exceeded. The treatment is performed in an outpatient service under sterile conditions in an operating room. After cleaning the skin around the eye, a local anesthetic with proxymetacaine hydrochloride 0.5 % eye drops is administered, an eyelid speculum is applied and the patient is placed under the laser (Schwind Amaris 750 S). First, the laser ablation is performed with the help of the laser software. Then the cross-linking procedure begins with the instillation of riboflavin eye drops for 15 minutes, followed by UVA light irradiation according to the accelerated CXL protocol (9 mW/cm² for 10 minutes). The postoperative medication corresponds to that described above (see section Standard CXL protocol) In the following, two cases of KC patients with combined treatments of CXL and transPRK are described.
Case 1
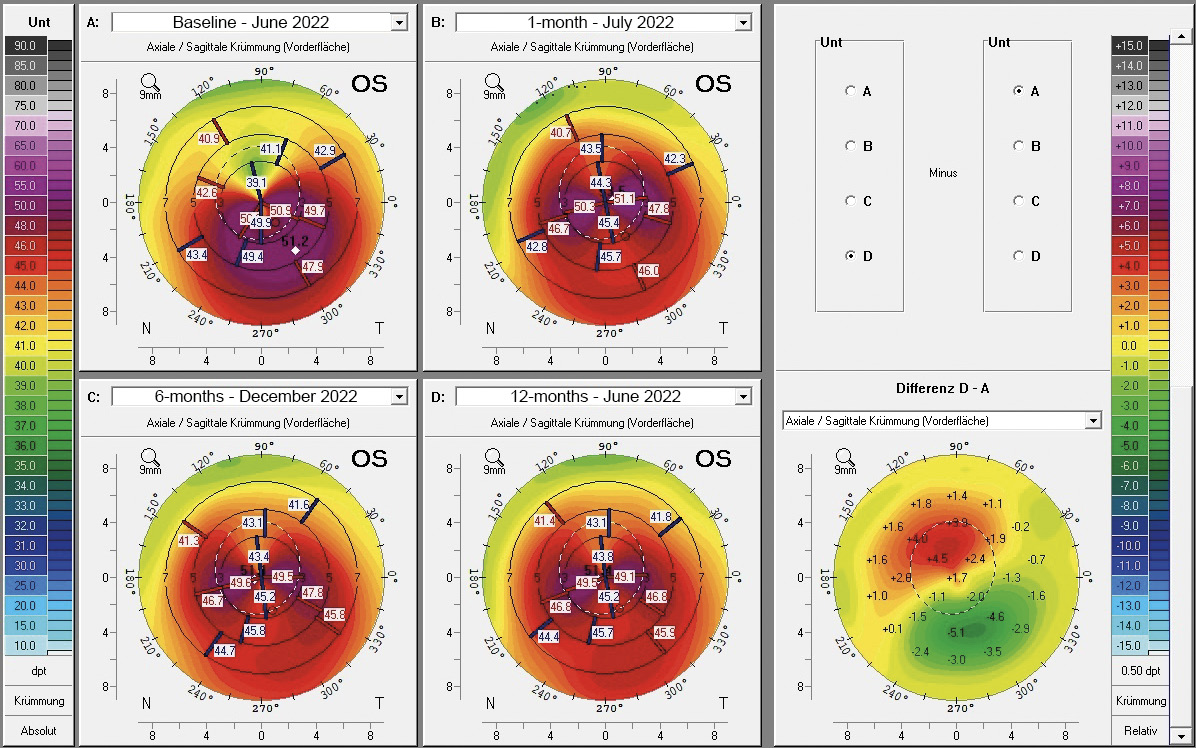
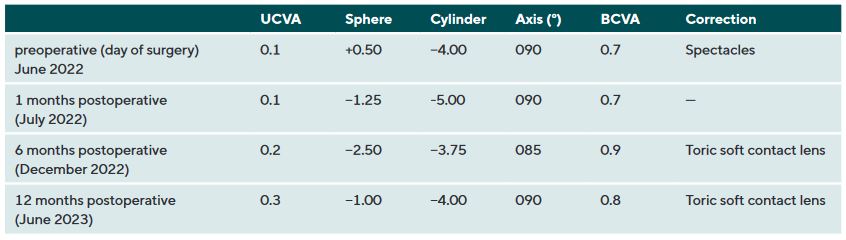
In July 2021, a 23-years-old female patient presented at the keratoconus KC clinic of the University Eye Hospital Carl Gustav Carus Dresden. She complained about headaches, vision problems during night driving, and hay fever. She had an unsuccessful RGP lens fitting due to an elevated foreign body sensation. The tomography (Pentacam HR, Oculus Optikgeraete GmbH, Wetzlar, Germany) of the right eye was normal despite the decreased corneal thickness and the decentered location of the thinnest point. The uncorrected and best-corrected visual acuities were 20/200 and 20/20, respectively, with a manifest refraction of +0.25 −1.00 × 070. On the left eye, a clinical KC was shown with BCVA of 20/30 and manifest refraction of +0.50 −3.50 × 095. At the 1-year follow-up (May 2022), the patient had progressed by more than 1 D in the left eye, where a CXL treatment was indicated in June 2022. She received a combined treatment of transPRK and CXL using the 9 mW/cm² / 10 min protocol. For the laser ablation, only high-order aberrations were corrected based on corneal topography measurement (Keratron Scout; Optikon 2000, Italy) by using an ablation zone of 7 mm. After treatment, the corneal topography showed a more regularized pattern with steep but regular astigmatism gainst the rule at 1 month, 6 months, and 12 months (figure 2). Topographic indices and root mean square of high-order aberrations were reduced after 1, and up to 12 months. The uncorrected visual acuity increased from 20/200 to 20/60 after 12 months (table 1). In addition, BCVA increased by 1 line after 12 months (table 1). At the 6-month follow-up, the patient was successfully fitted with a soft toric contact lens with a base curve of 8.6 mm, a power of −2.50 −3.75 × 085, and a diameter of 14.2 mm (Contact Individual Bio TD, Wöhlk Contactlinsen GmbH, Germany).
Case 2
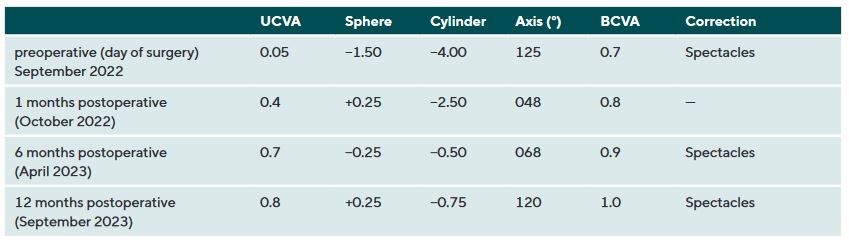
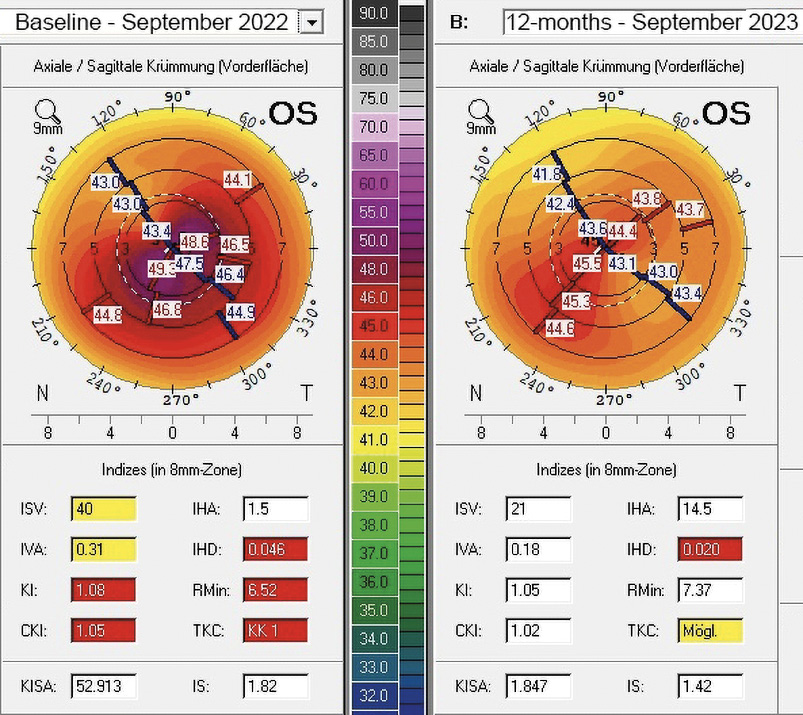
A 31-years-old male patient presented at the KC clinic of the University Eye Hospital Carl Gustav Carus Dresden in March 2022. He complained about distorted vision which did strongly affect his daily vision. Corneal tomography was normal in the right eye, where the uncorrected and best-corrected visual acuities were 20/25 and 20/20 (refraction Pl. −0.50 × 025), respectively. In the left eye, a symmetric bow-tie pattern (in 44°) with increased central corneal curvature appeared in corneal tomography with a decreased corneal thickness (thinnest point was 495 μm, figure 3, left). The uncorrected and BCVA was 20/400 and 20/30 (Rx −1.25 / −3.25 / 130°), respectively. A progression of the KC was observed in the left eye during the follow-up examinations, where a CXL procedure was indicated. The patient required better vision for professional reasons, as he wanted to become a truck driver. The patient received transPRK and CXL treatment to correct refraction and high-order aberrations by the laser procedure. Postoperatively, a regularization of the anterior corneal curvature with a reduction in topography indices, corneal astigmatism, and root mean square of high-order aberrations could be shown (figure 4). Vision improved from 20/400 to 20/25 and from 20/30 to 20/20 for uncorrected and BCVA after 12 months. The patient was satisfied with the spectacle correction (table 2).
In conclusion, the combination of CXL and transPRK is a promising technique for optimizing distorted vision in mild to moderate KC patients. The current indications for treatment are: Kmax below 55.0 D, stromal ablation in the cone area below 50 μm, and progression of KC. The procedure aims to stabilize the cornea and improve vision with glasses or soft contact lenses when RGP lenses are not tolerated. Spectacle-free or contact lens-free vision is not the primary goal.
The customization of UVA light irradiation
The corneal (stromal) tissue is biomechanically weakened focally in KC, as mentioned above.9 This fact was also confirmed recently using Brillouin microscopy.48,49 This raises the question of treating only the weakest part of the cornea with a higher fluence than the outer area with more normal biomechanical properties. New UVA light devices for CXL treatments allow the cornea to be irradiated decentrally and focally, using higher fluence (more than 5.4 J/cm²) in the weakest area and standard fluence (5.4 J/cm²) in the outer area. Studies have shown that the point of the maximum corneal elevation on the posterior surface of the cornea might be the best focal point representing the weakest part of the cornea.48 Seiler et al. reported the first clinical results using the customized protocol with a 1-year follow-up. They showed a significantly stronger decrease of anterior keratometry values in the customized CXL group compared to the standard protocol. Concerning the rates of flattening, 52.6 % had a decrease of more than 1.0 D in the customized CXL group, whereas 36.8 % flattened by 1 D in the standard CXL group.50 Another study from India presented similar data regarding the comparison between customized and accelerated CXL. They found a flattening (more than 1 D) of the cornea in 96.8 % of the treated patients, whereas only 55.3 % flattened in the standard CXL group. At the same time, high-order aberrations, especially coma, were significantly reduced leading to an improvement in BCVA.
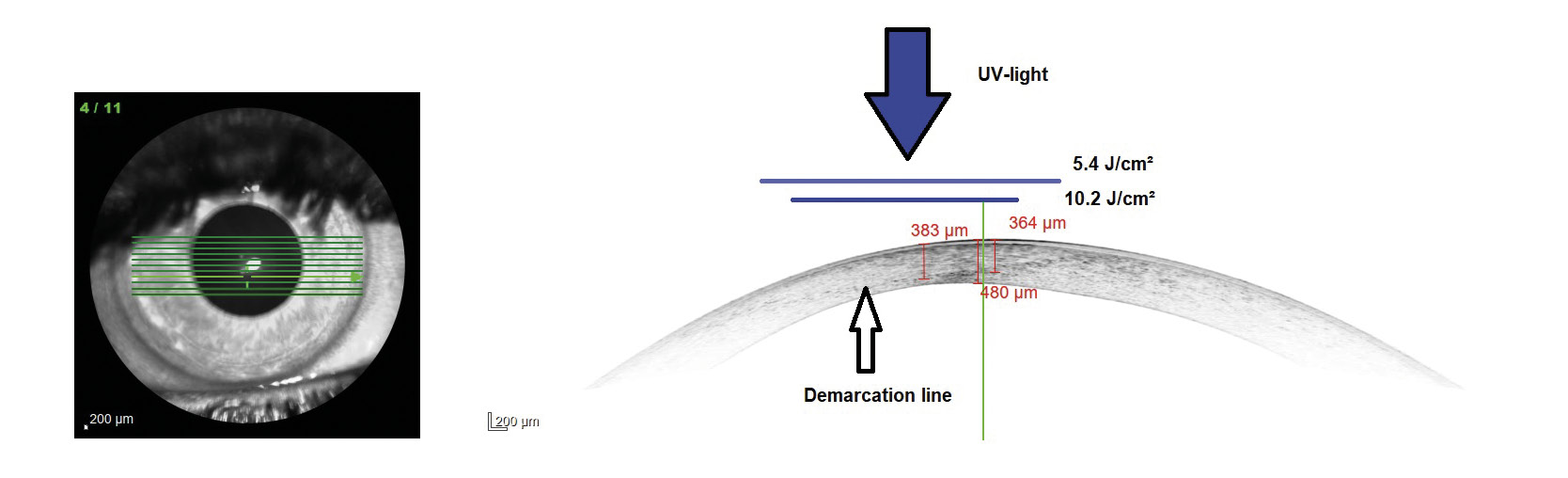
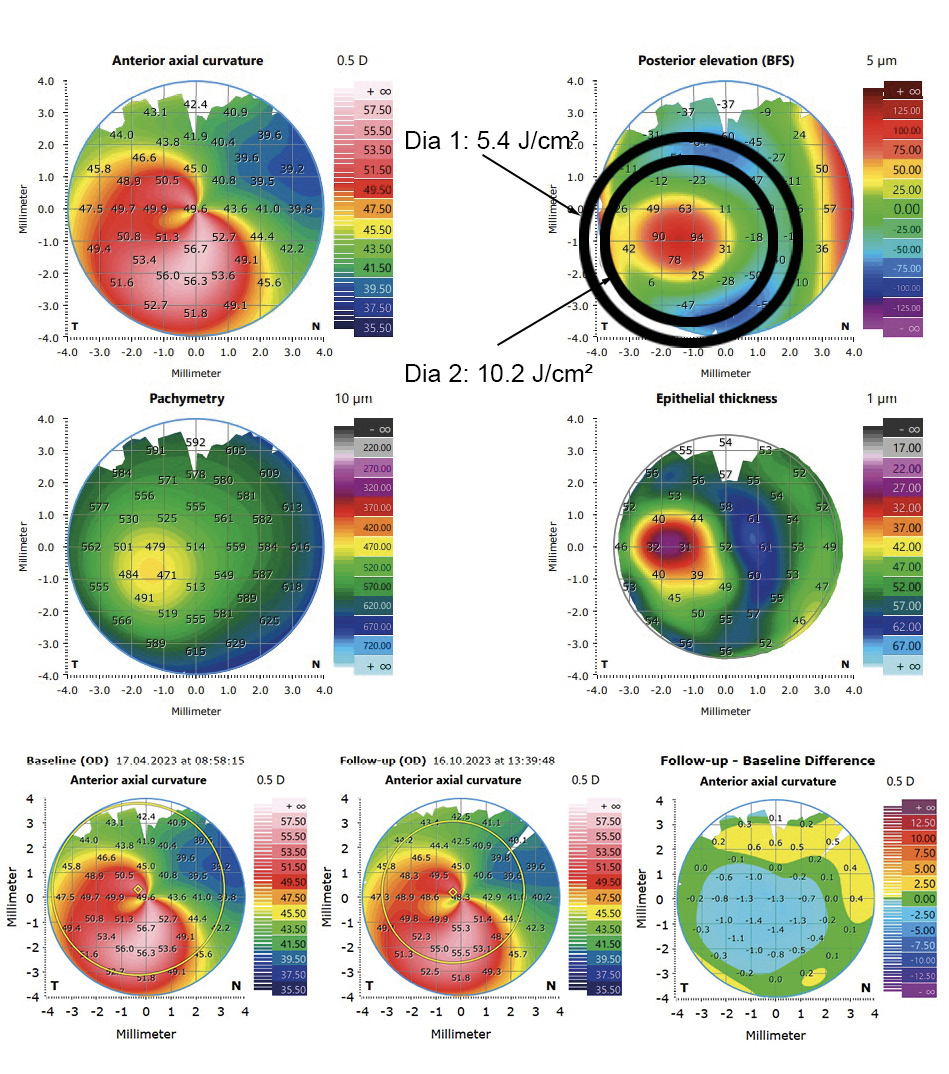
These results can be explained by the fact that the higher fluence in the area of the cone lead to a deeper CXL effect in the corneal tissue without damaging endothelial cells. A modified protocol is used for customized CXL treatments at the University Eye Hospital Carl Gustav Carus Dresden, where the cornea is irradiated with two different diameters depending on the area of the cone. The UVA light is centered on the maximum posterior elevation as proposed by Seiler et al. The modified protocol is similarly effective which can also be confirmed by the so-called demarcation line. This line qualitatively evaluates the depth of CXL, where the transition zone between the treated and untreated area of the tissue is presented. In customized irradiation patterns, the demarcation line is deeper in the area where the fluence is the highest (figure 5). In figure 6, an example case is shown. The UVA light diameter is centered over the maximum posterior elevation with the larger diameter (DIA 1) irradiated with 5.4 J/cm² and the smaller diameter (DIA 2) with 10.2 J/cm² (upper part). In the lower part, the change in corneal curvature is displayed between baseline and 6 months after treatment. The reduction in the treated area is up to −1.4 D. The long-term follow-up will show whether further corneal regularization takes place and whether visual acuity improves as a result.
Conclusion
Corneal CXL is an established treatment for stopping the progression of KC. The standard and accelerated protocols effectively stabilize the cornea for long periods of time with low rates of re-progression. Newer protocols aim to improve visual acuity by regularizing the irregular cornea. This also leads to better spectacle-corrected visual acuity or contact lens fitting.
Financial disclosure
This article did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
