Fast Progression to Proliferative Diabetic Retinopathy
Purpose: This case report illustrates rapid progression of diabetic retinopathy in a patient with poor compliance to diabetic management. It additionally demonstrates the utility of panoramic/ultra-wide field retinal imaging in assessing diabetic retinopathy progression.
Material and Methods: A 42-year-old female patient was examined seven times over a 25 month period at a private ophthalmology/optometry practice with dilated fundus examinations. Fundus images were obtained using a panoramic/ ultra-widefield laser retinal imager. Fluorescein angiography (FA) was performed to assess for diabetic macular edema (DME), retinal ischemia and neovascularization, and laser photocoagulation was conducted as indicated by the clinical picture. Ophthalmic ultrasonography was performed to confirm tractional retinal detachments (TRD) at the final visit.
Results: Initially, visual acuity was adequate (20/30; 6/9; LogMAR 0.18 in each eye) and the patient was diagnosed with moderate non-proliferative diabetic retinopathy. Diffuse leakage resulting in DME, confirmed with FA at the second visit, was treated with focal laser photocoagulation. However, after being lost to follow-up for over a year, her vision had deteriorated severely to finger counting at 1ft (30.5 cm) in the right eye, and 20/400; 6/120; LogMAR 1.30 in the left: the result of high-risk proliferative diabetic retinopathy in each eye. The patient underwent three treatments of pan-retinal photocoagulation (PRP), but she nevertheless developed tractional retinal detachments (TRD) in both eyes.
Conclusion: The combination of poorly controlled diabetes and poor compliance with follow-up care in diabetic retinopathy can lead to rapid progression of retinopathy and blindness. Early detection of diabetic retinopathy with panoramic/ ultra-wide field retinal imaging allows for appropriate staging and management of the disease, and ultimately, better visual outcome.
A 42-year old myopic Hispanic female presented for a contact lens check-up. She reported mild blurred vision in each eye, a history of Type 2 (non-insulin-dependent) diabetes mellitus (NIDDM) diagnosed 6 years earlier, and systemic hypertension, both of which were being treated by her internist with “several pills” (medication names were unknown to the patient). She claimed a recent blood glucose measurement of 200 mg/dL and blood pressure of 130/80 mmHg. The patient reported that she was having difficulty controlling her weight, which varied between 200-220 lbs (90.7 – 99.8 kg); in this 5’3” (160cm) female, her body mass index (BMI) therefore varied between 35.4 and 39.0, defining her as clinically obese per World Health Organization (WHO) guidelines.1
Two pathophysiological factors lead to retinopathy and reduced vision in uncontrolled diabetics as a result of prolonged hyperglycemia: macular edema caused by leakage of blood products from microaneurysms that form in response to weakened capillaries, and retinal ischemia caused by a thickened basement membrane and other factors decreasing the size of the capillary lumen.2 This ischemia can further lead to neovascularization of the retina and optic disc, which can cause extensive pre-retinal and vitreous hemorrhaging, as well as tractional retinal detachments due to fibrovascular proliferation, both of which can result in severe vision loss. Both the American Optometric Association and the American Academy of Ophthalmology recommend annual dilated fundus examinations for all patients diagnosed with diabetes in order to rule out ocular signs of leakage (hemorrhaging of microaneurysms with associated exudation and edema) and ischemia (cotton wool spots (CWS), venous beading (VB), intra-retinal microvascular anomalies (IRMA)), as well as neovascularization of the disc (NVD) and elsewhere (NVE).3,4 Careful examination of these findings allows the practitioner to stage diabetic retinopathy per the Early Treatment of Diabetic Retinopathy Study (EDTRS) and international disease severity scales.3-6
The ETDRS defined mild non-proliferative diabetic retinopathy (NPDR) by the presence of microaneurysms, moderate NPDR as retinopathy with signs greater than microaneurysms alone but not yet meeting the definition of severe NPDR, and severe NPDR as the presence of 4 quadrants of severe intra-retinal hemorrhages, 2 quadrants of venous beading, or 1 quadrant of IRMA.5 The hallmark of Proliferative diabetic retinopathy (PDR) is the presence of NVD or NVE, and while the standard of care for NPDR is observation and education on the importance of tight glycemic control, PDR can be treated with pan-retinal photocoagulation (PRP) and/or anti-angiogenic agents.3-6 Diabetic macular edema (DME) can occur at any stage of retinopathy due to leakage of microaneurysms, and the ETDRS defined “clinically significant” macular edema (CSME) as retinal thickening (or exudates associated with adjacent thickening) that falls within 500m of the macular center; focal laser photocoagulation was found to improve visual outcomes in patients with CSME. In the past decade, a paradigm shift for DME treatment has occurred as a result of the wide adoption of anti-angiogenic agents, and the term “center-involved” diabetic macular edema (ciDME) has largely replaced CSME in the United States.3,4,6 It is a more general term, describing any thickening within the center 1mm subfield of the retina, retaining the 500m radius from the CSME definition but foregoing any specific reference to exudation or area of edema.
Appropriate diagnosis and staging of diabetic retinopathy is essential for establishing treatment strategies and follow-up scheduling, as visual outcomes depend on effective management. However, visual outcomes also depend on patients’ glycemic control and compliance to treatment and follow-up; this case shows the potential consequences of noncompliance.
Material and Methods
The patient was examined seven times over a 25 month period with dilated fundus exams at a private ophthalmology/optometry practice in New York State, USA. The first two exams were one month apart, but the patient was then lost to follow-up and her third exam was 17 months after the second; the remaining four were all within 1-2 months of each other. Visual acuities were measured using a digital Snellen eye chart. Fundoscopy was performed using a 90D lens and slit lamp biomicroscope, and images were obtained at initial and all subsequent visits by an Optos P200MA panoramic/ultra-wide field laser retinal imager (Optos PLC, Dunfermline, Scotland (United Kingdom)) with Optomap® color and green separation (“red-free”) imaging modalities. Fluorescein angiography (FA) was performed as necessary to assess for diabetic macular edema (DME), retinal ischemia and neovascularization using the Optomap® fa imaging modality of the Optos P200MA laser retinal imager. Laser photocoagulation (both focal and pan-retinal) was performed at the same private practice as indicated by the clinical picture, and ultrasonography was performed to confirm tractional retinal detachments with a Sonomed E-Z Scan AB5500+ (Sonomed Escalon, New Hyde Park, NY).
Results
Initial Visit (V1)
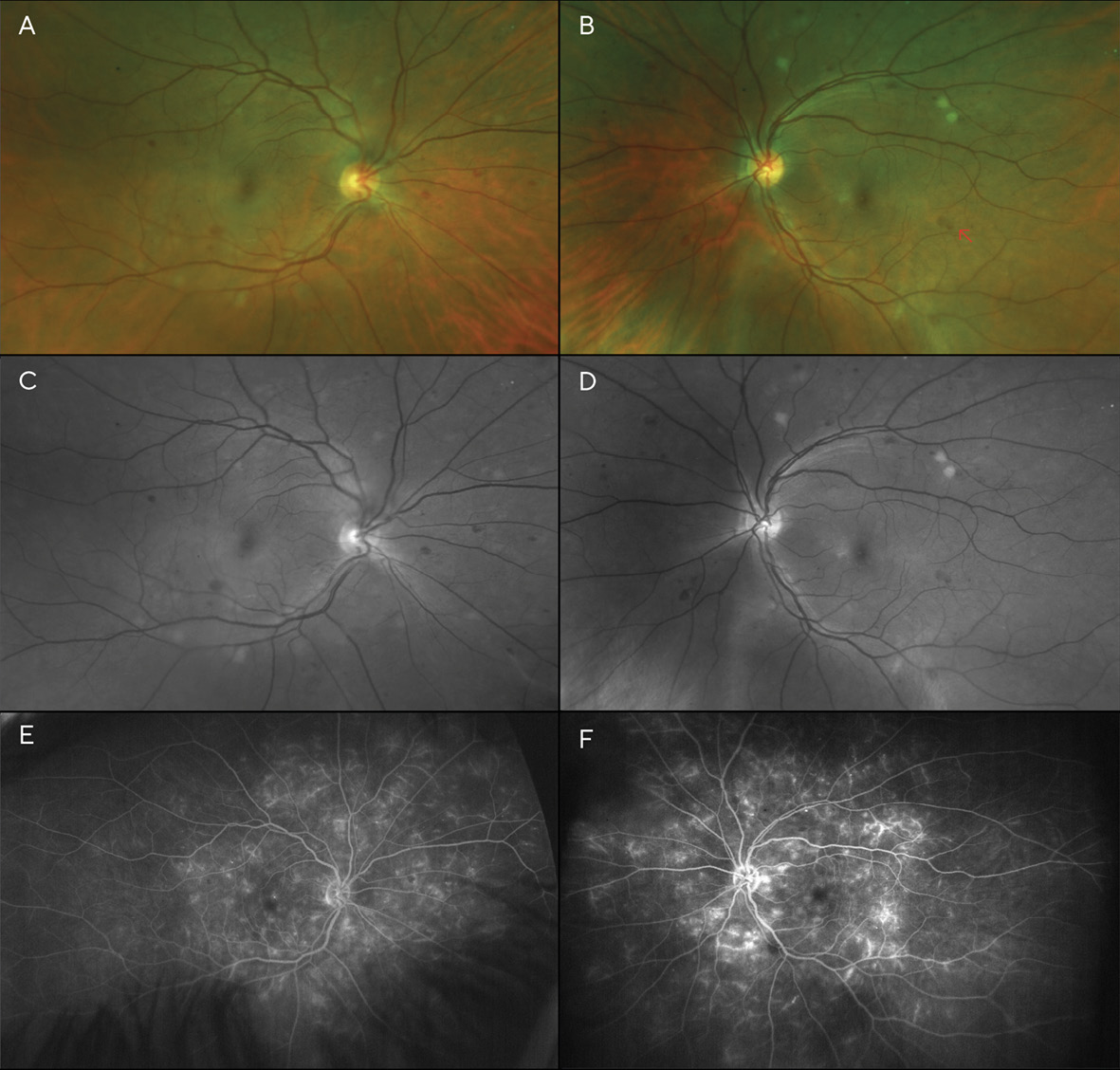
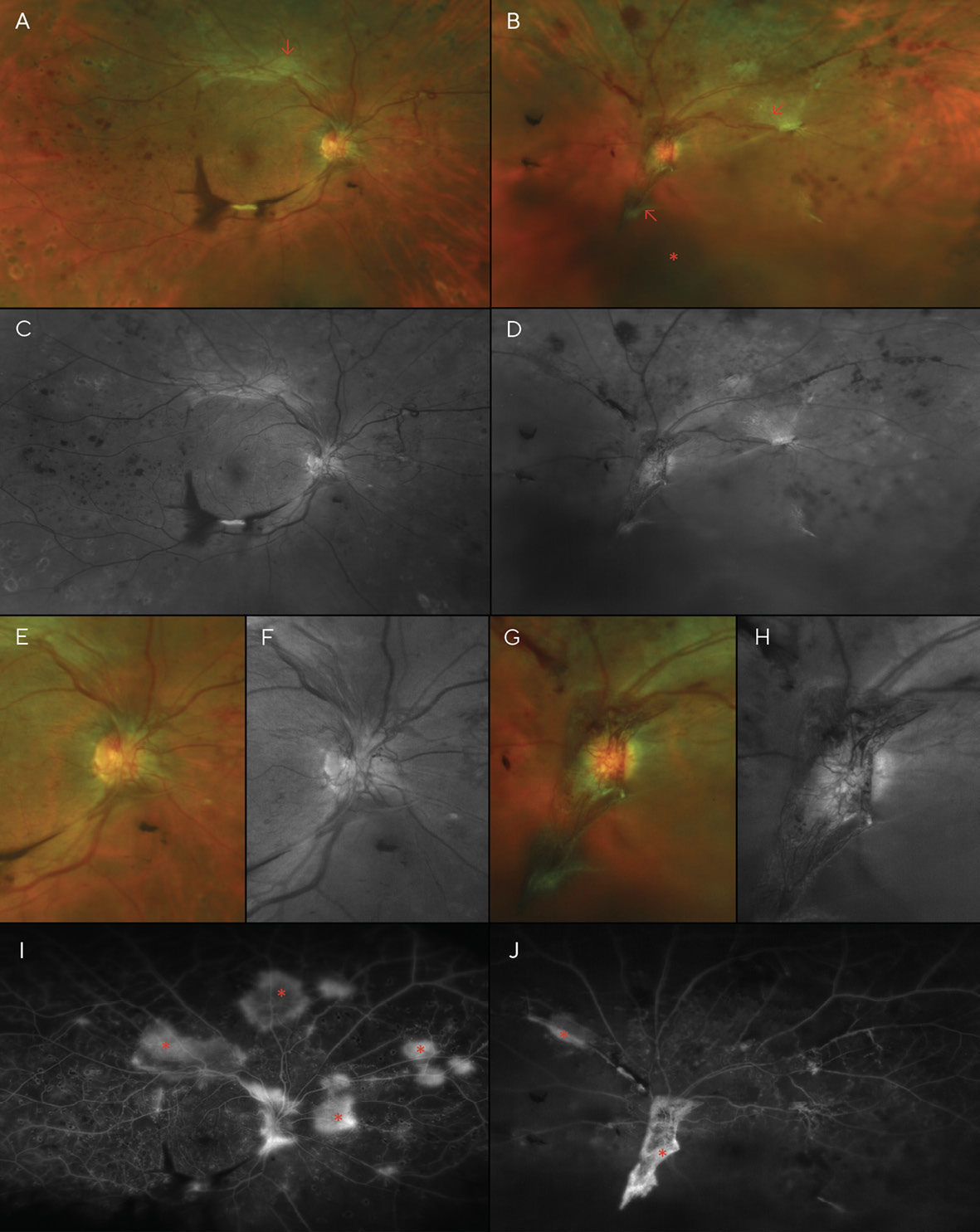
Best corrected visual acuities (BCVA) at the patient’s initial visit were 20/30 (6/9; LogMAR 0.18) in each eye with a -5.00sph refraction OD and OS. Fundoscopy revealed scattered dot and blot hemorrhages and several cotton wool spots in both eyes—though more pronounced in the left eye (Figures 1A-1D). Additionally, the left fundus showed an area of possible, mild IRMA (intra-retinal microvascular anomalies), temporal to the macula. Based on the clinical findings, the diagnosis of moderate non-proliferative diabetic retinopathy (NPDR) was made, and the patient was instructed to return in one month for a follow-up appointment, including fluorescein angiography to evaluate for retinal ischemia and leakage. The patient was also educated on the importance of good glycemic control with medication, diet, and exercise, with regular visits to her internist.
Visit 2: 1 month after V1
The fluorescein angiogram was performed a month later; entering visual acuity was unchanged at this visit (20/30 (6/9; LogMAR 0.18) in each eye). It revealed diffuse leakage in both eyes (Figures 1E & 1F). Diffuse, center-involved diabetic macular edema was diagnosed, and treatment with focal laser in the para-macular area was recommended and performed in each eye one month later. The patient admitted to non-compliance with her diabetic medications; she was not taking them as often as they were prescribed. She was educated about the risk of vision loss with progression of her retinopathy, and tight control of her blood glucose level and blood pressure were stressed. A 2-month follow-up was scheduled after the laser treatments were performed in both eyes.
Visit 3: 18 months after V1
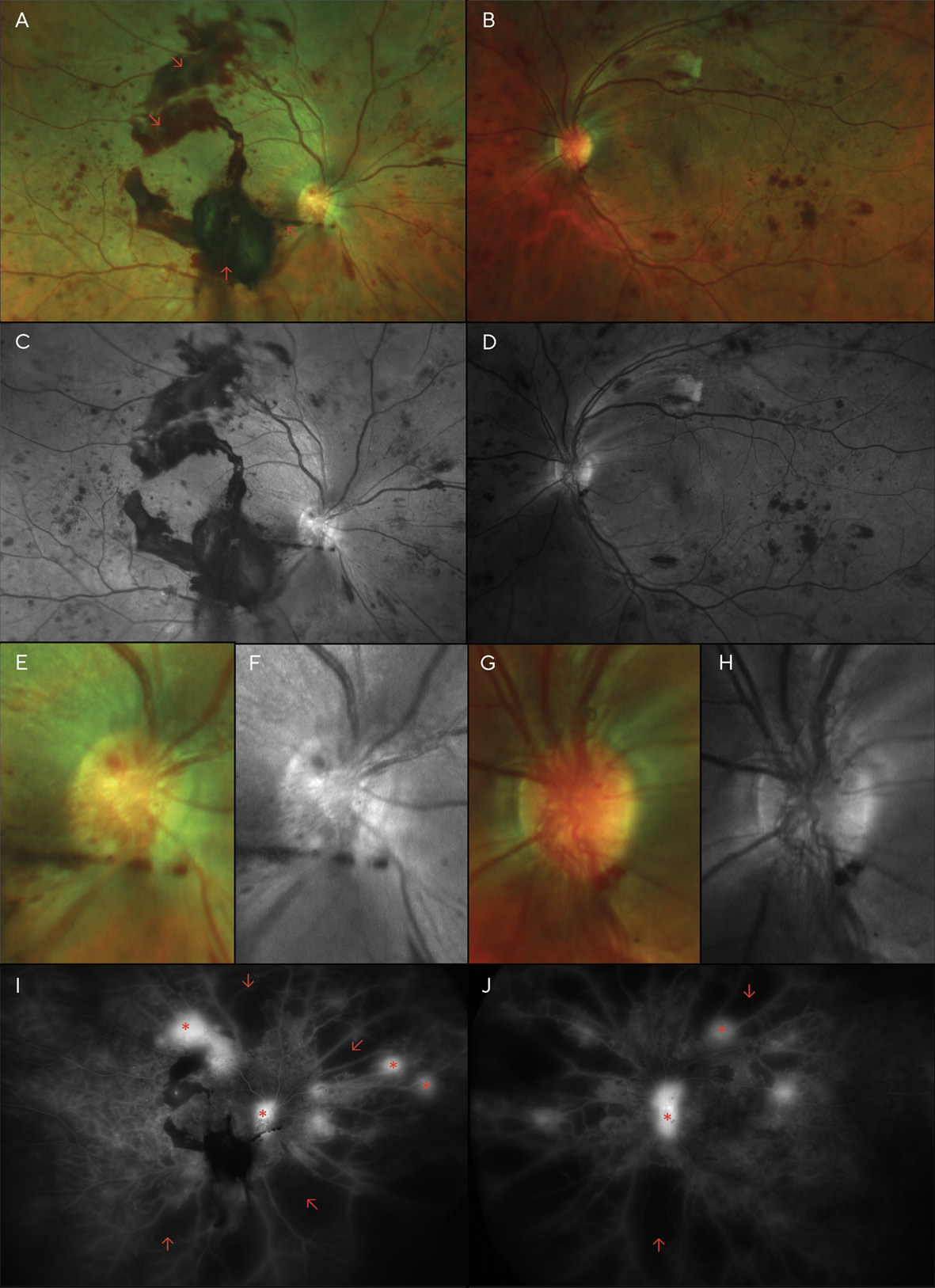
Although the patient was scheduled for an appointment 2 months after her focal laser treatments, she was lost to follow-up for over a year and failed to return even after mail and telephone recall. Instead, she returned 16 months later, complaining of moderately blurred vision over the previous several months in both eyes and sudden vision loss in the right eye that began several days earlier. She again reported her blood glucose level to be around 200 mg/dL, and she admitted to non-compliance with medications. Examination revealed acuities of Finger Counting at 1 ft (30.5 cm) OD, and 20/400 (6/120; LogMAR 1.3) OS. Dilation revealed several areas of pre-retinal (sub-hyaloid) and vitreous hemorrhaging, much of which overlies the macula of the right eye, likely the cause of her sudden vision loss. Additionally, marked dot and blot hemorrhages were noted in all 4 quadrants of each eye, as well as cotton wool spots and neovascularization of the disc (NVD) and elsewhere (NVE) in both eyes (Figure 2).
A diagnosis of high-risk proliferative diabetic retinopathy (PDR) was made, and pan-retinal photocoagulation (PRP) was performed in each eye over the next several weeks.
Visit 4: 20 months after V1
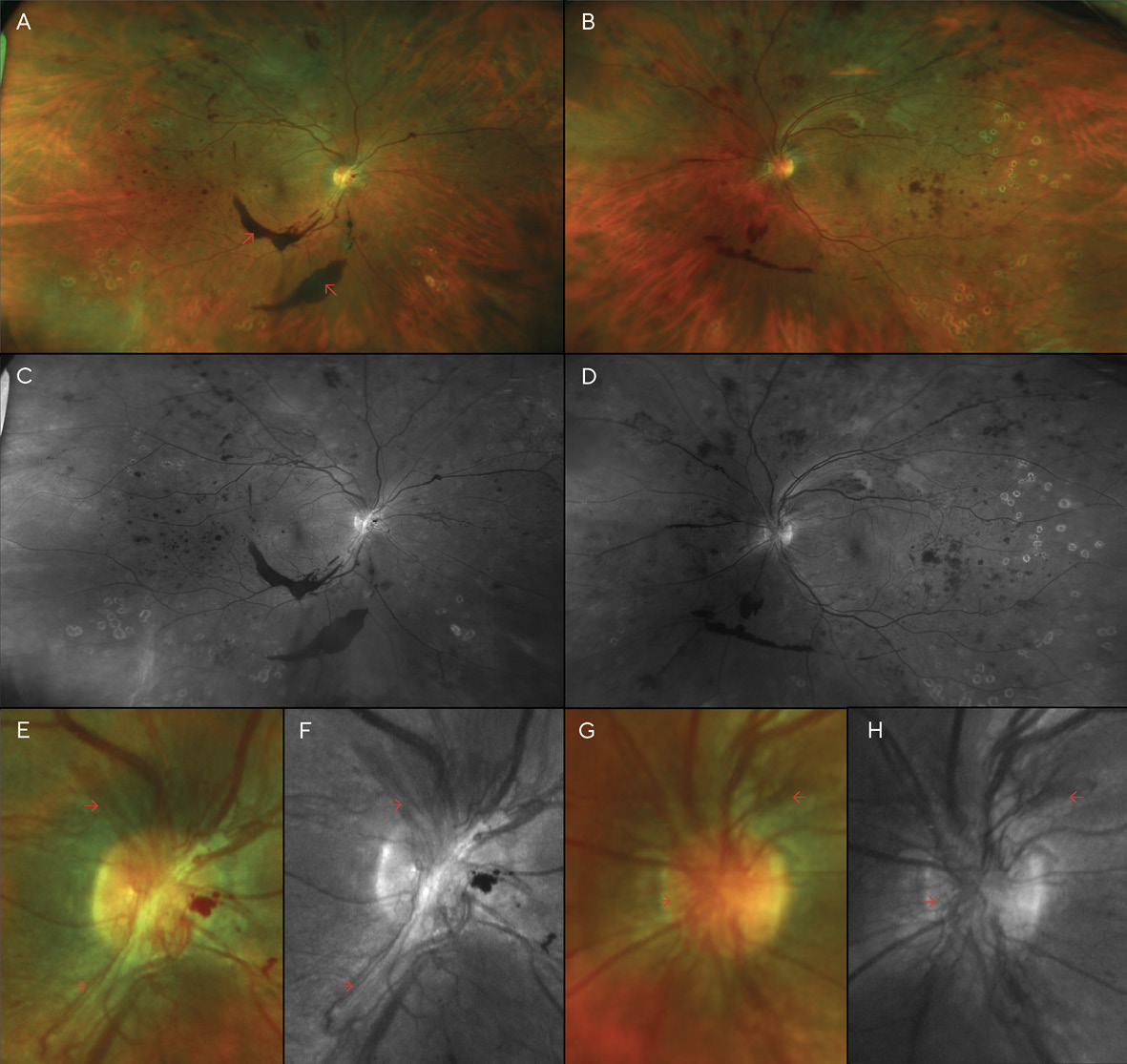
After PRP was performed in both eyes, the patient returned for a follow-up, where BCVA had improved to 20/70 (6/21; LogMAR 0.54) in each eye; dilation revealed that the pre-retinal hemorrhage of the right eye had settled to below the visual axis, allowing the patient to see more clearly in that eye for the first time in several months. However, despite the PRP treatments, the patient’s NVD & NVE had worsened (Figure 3). Thus, additional PRP was recommended and performed in each eye. Careful slit lamp exam and gonioscopy failed to reveal any neovascularization of the iris or angle. At this time, medical records were requested of the patient’s internist after the patient authorized their release. Review of these records indicated that glucose levels had reached 460 mg/dL 13 months after V1, and the patient’s internist added insulin to her treatment regimen at that time. Additionally, Hemoglobin A1C was noted to be above 10.0% on several visits.
Visit 5: 21 months after V1:
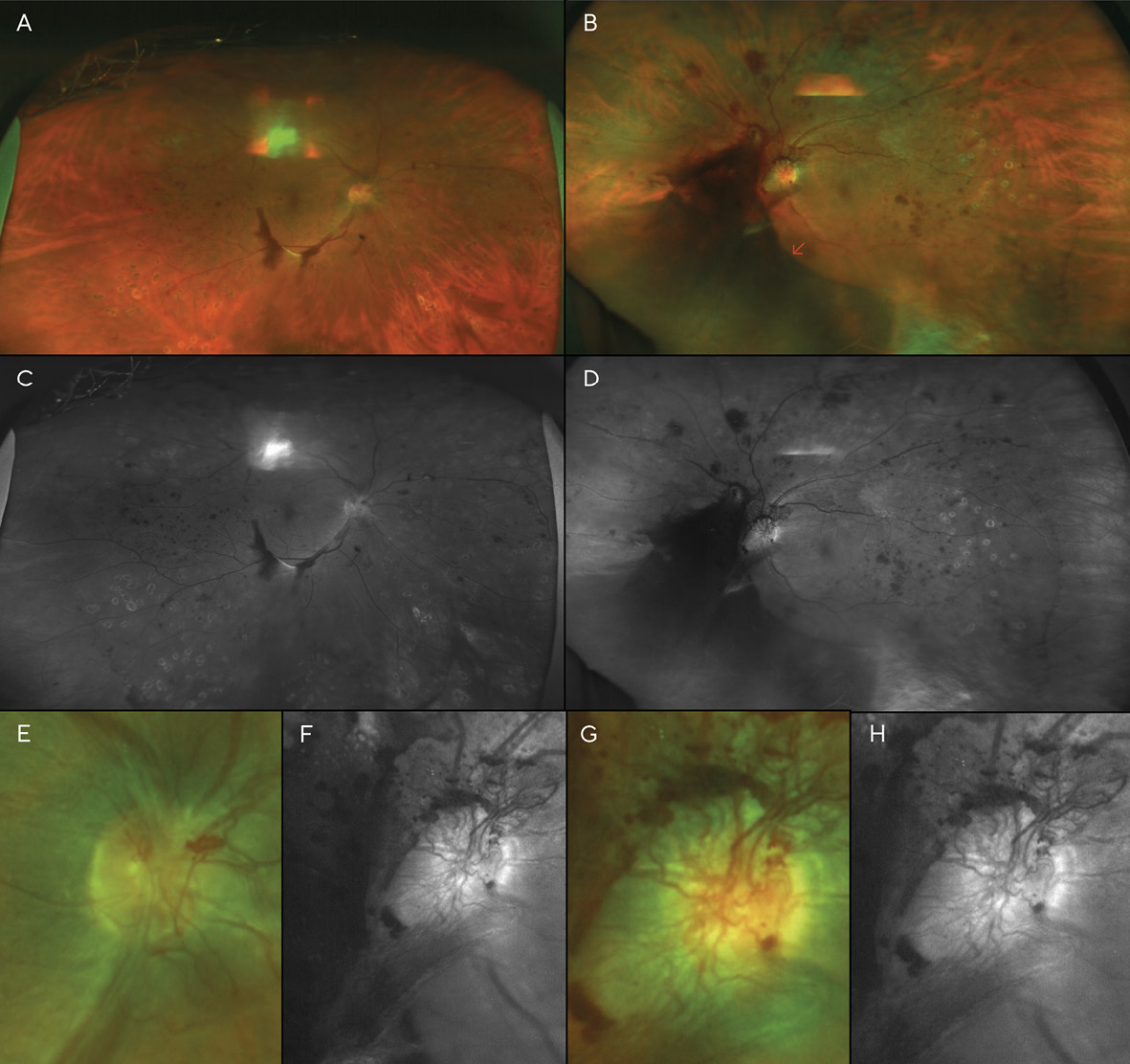
A month later, a large vitreous hemorrhage was noted inferonasal to the optic disc and macula of the left eye (Figure 4). Additional PRP was again recommended and provided in each eye. At this point, the patient had received a total of three (3) PRP treatments in each eye.
Visits 6 & 7: 22 & 25 months after V1
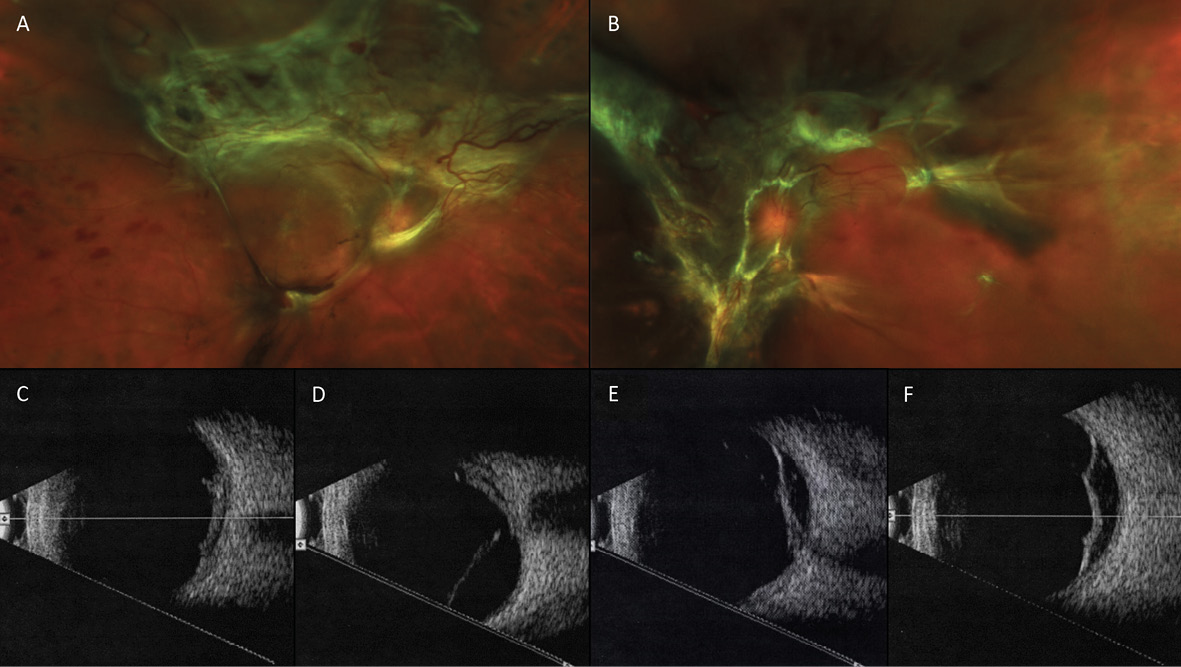
A month after the third PRP procedure, the inferior vitreous hemorrhage of the right eye was beginning to resolve (Figure 5). However, 3 months after this visit, fibro-proliferative tissue that provided scaffolding for the NVD and NVE had created traction on the retina, resulting in tractional retinal detachments (TRD) in both eyes (Figure 6). B-scan ultrasonography confirmed this finding.
Vascular endothelial growth factor inhibiting (anti-VEGF) intravitreal injections were discussed with the patient previously for treatment of her PDR, but she refused. However, after explaining the fast, 3-month progression of her retinopathy to the patient—with the aid of the wide-angle fundus images—the patient agreed to Avastin® treatments (Genentech, San Francisco, CA) and additional PRP.
Discussion
Diabetic retinopathy is the leading cause of blindness among adults aged 20-74 years; among diabetics, estimated global prevalence of any diabetic retinopathy (DR) and proliferative diabetic retinopathy (PDR) is 35.4% and 7.5% respectively. 7,8 In the United States, where the 42-year old patient in this case report resides, prevalence of retinopathy in diabetic patients over 40 years old is 28.5%.9
Though the staging and classification of diabetic retinopathy was established in the 1980’s and 90’s by the landmark Early Treatment of Diabetic Retinopathy Study (ETDRS), the classifications remain today as useful indicators of disease progression and as a guide for follow-up schedules.3-7,10 The patient in this case report presented with moderate non-proliferative diabetic retinopathy (NPDR)—defined in the ETDRS as the presence of marked intraretinal hemorrhages and/or microaneurysms and/or signs of ischemia such as cotton wool spots, mild venous beading, or mild intraretinal microvascular abnormalities (IRMA) that don’t meet the criteria to be classified as severe NPDR.5 A study on five-year progression patterns on diabetic retinopathy in the United States revealed that the incidence of progression to PDR—marked by neovascularization of the disc (NVD) or elsewhere (NVE)—rises with the level of baseline retinopathy.11 Of patients with moderate NPDR at baseline, 17.6% progress to PDR and 44.6% develop diabetic macular edema (DME) within 5 years. However, the patient in this case report progressed from moderate NPDR to PDR within a span of only 16 months and presented with diffuse DME at her initial visit. A variety of factors contribute to progression in diabetic retinopathy, including glycemic control, duration of diabetes, and the presence of hypertension and/or hyperlipidemia. It has been well-established that glycemic control is correlated to the level of diabetic retinopathy; the Diabetes Control & Complications Trial (DCCT), conducted in the United Kingdom from 1982-1993, was the first major trial to quantify the protective effect that tight blood sugar control has on the development of end-organ damage (retinopathy, nephropathy, neuropathy).12,13 Patients were randomized to receive either intensive or conventional therapy for their insulin-dependent diabetes mellitus (IDDM; “Type 1” diabetes mellitus). After 5 years, the intensive treatment group showed a 50% risk reduction in retinopathy, and the DCCT established a clear relationship between Hemoglobin A1C and risk of retinopathy. Similar findings were noted in the United Kingdom Prospective Diabetes Study (UKPDS), which examined the effects of intensive control on patients with non-insulin-dependent diabetes mellitus (NIDDM; “Type 2” diabetes mellitus).14 It should be noted, however, that a quick tightening of poor diabetic control can sometimes have the paradoxical effect of a transient worsening of retinopathy before later improvement.15 The reasons for this phenomenon are still an active area of research, but hypotheses of osmotic pressure changes, synergy between insulin and VEFG on retinal blood vessels, blood-retinal barrier breakdown, and VEGF upregulation have all been proposed.
The patient in this case study admitted to poor glycemic control, and medical records review revealed A1C >10.0% and blood glucose levels above 400. Additionally, the patient’s internist had added insulin to her treatment regimen, a sign of a poorly controlled NIDDM patient; medical therapy for well-controlled NIDDM is often oral antihyperglycemic medications alone. Her poor diabetic control—and possibly the need for intensive treatment with insulin—was likely a factor in this patient’s relatively fast conversion from moderate NPDR to PDR.
Additional risk factors for retinopathy progression in diabetics include both high blood pressure and high blood cholesterol.16-20 While blood cholesterol levels were unknown for this patient and she claimed a blood pressure of 130/80, she was treated for systemic hypertension and admitted to non-compliance with medications for both diabetes and hypertension. A systolic blood pressure of less than 140mmHg is recommended to decrease the likelihood of retinopathy progression6; one study recommends lowering blood pressure below 130/75 for any patient showing retinopathy.20 Not only are hypertension and hyperlipidemia correlated with retinopathy progression, they are similarly linked to increased chances of nephropathy as well; incidence of kidney disease requiring dialysis and/or transplant is higher in hypertensives than those with normal blood pressure.21,22 Additionally, a linkage between the presence of diabetic retinopathy and renal disease has long been recognized. One such study on NIDDM in white males showed that 76% of the patients who progressed to diabetic nephropathy also had diabetic retinopathy.22 Since loss of kidney function could lead to hospitalization and death, and the risk factors for diabetic retinopathy progression are equivalent to those for nephropathy, finding signs of retinopathy early can save not only a patient’s vision, but possibly their life, too.
While obesity is closely associated with increased risk of NIDDM—85.2% of diabetics are overweight; 52% are obese23—a meta-analysis and systematic review of 27 articles did not establish obesity nor being overweight as a risk factor for retinopathy in diabetics.24 The patient in this case was clinically obese, which may have been a major contributor to her original development of NIDDM, but likely was not a factor in progressing from moderate NPDR to high-risk PDR so quickly.
Conclusions
In this case, the patient progressed from moderate NPDR to PDR with bilateral tractional retinal detachments in just 1.5 years; her poor compliance with her diabetes treatment regimen and follow-up schedule was likely the primary factor in this fast progression rate. Because diabetic nephropathy is highly correlated with diabetic retinopathy, early detection of diabetic retinopathy with dilated fundus examinations, panoramic/wide-angle fundus imaging and/or spectral domain optical coherence tomography (OCT) is equivalent to early detection of diabetic nephropathy and may result both in better visual and systemic outcomes with timely intervention. Practitioners must educate patients on the importance of tight blood glucose, blood pressure, and cholesterol control, as well as compliance with follow-ups and visits to their primary medical care provider for treatment and management of their systemic disease. Since compliance not only with follow-up visits but also with glycemic control was likely the main factor in this patient’s progression, new methods to improve medication and follow-up compliance among patients should be explored. These could include automated text messaging or email appointment reminders, and a renewed emphasis on communication with internists, dieticians, and endocrinologists who manage patients with diabetes. The risk of blindness must be stressed, particularly in patients already presenting with some form of diabetic retinopathy, as these patients are more likely to progress. The combination of poorly controlled diabetes and poor compliance to follow-up schedules in diabetic retinopathy can lead to rapid progression and blindness which is often preventable.
Conflict of interest
The primary author has nothing to disclose. The secondary author, Dr. Jerome Sherman, has served as a consultant to Optos PLC, but not in the past 5 years. However, he received no funding nor has no financial interest in this case report. This case was previously presented as Case #41 on Dr. Sherman’s “retina revealed” website (www.retinarevealed.com).
Acknowledgements
We acknowledge Sheikh Hussein, MD, who performed the fluorescein angiography for the patient in this case report, and Sanjeev Nath, MD, who performed the focal and pan-retinal photocoagulation procedures.
