Rhopressa Clinical Use: A Case Series
Purpose: The aim of this paper is to discuss and highlight via case reports the effective use of Rhopressa in three individual cases of Primary Open-Angle Glaucoma (POAG) treated at a glaucoma clinic in Philadelphia, PA, USA. The cases display the clinical use of netarsudil and reviews therapeutic approaches to combating the side effects of conjunctival hyperemia associated with netarsudil.
Material and Methods: A systematized PubMed literature search based on the terms “Rhopressa”, “netarsudil”, “ROCK inhibition” was conducted. Six eyes of three adult patients were monitored for three months after beginning treatment with Rhopressa (0.02% netasurdil ophthalmic solution, Aerie Pharmaceuticals Inc, Durham, NC, 2018).
Results: In all three cases, an efficacious response was achieved with all three patients obtaining an intraocular pressure of 13 mmHg or lower. In two out of three cases the patients were taking three or more medications prior to the addition of Rhopressa (0.02% netasurdil ophthalmic solution, Aerie Pharmaceuticals Inc, Durham, NC, 2018). Two eyes out of six possessed a stage of severe glaucoma and two out of six eyes previously underwent multiple surgical glaucoma interventions.
Conclusion: A desirable intraocular pressure reduction was found in all six eyes out of three patients with an acceptable tolerability of use of the medication. Rhopressa (0.02% netasurdil ophthalmic solution, Aerie Pharmaceuticals Inc, Durham, NC, 2018) is a viable and effective adjunct therapy for the treatment of open-angle glaucoma with adequate patient tolerability.
Introduction
Glaucoma is the leading cause of irreversible blindness worldwide. Current global prevalence of open-angle glaucoma is 3.54 % in populations 40-80 years old. As of 2013, the disease has been estimated to affect 64.3 million individuals with a 2020 projection increase to 76 million and 111.8 million by 2040 around the world.1 The only modifiable risk factor in the treatment of open-angle glaucoma is lowering of intraocular pressure (IOP). Our current medical armament includes prostaglandin analogues as our most efficacious and mainstay of first line treatment. Prior to Rhopressa (0.02% netasurdil ophthalmic solution, Aerie Pharmaceuticals Inc, Durham, NC, 2018), the last novel anti-glaucoma drug was the advent of prostaglandins. Xalatan (0.005% latanoprost, Pfizer Inc, New York, NY) was introduced in 1996 followed by other prostaglandin analogues including Lumigan (0.01% bimatoprost, Allergan Inc., Dublin, Ireland) and Travatan, (0.004% travoprost, Alcon Laboratories Inc, Ft Worth, TX) in the early 2000s.2 Current adjunct lines of treatment include three other classes of medications; carbonic anhydrase inhibitors, beta blockers, and alpha agonists. Rhopressa (0.02 % netasurdil ophthalmic solution, Aerie Pharmaceuticals Inc, Durham, NC, 2018) represents the first novel drug for IOP reduction in over 20 years. This medication was approved in the United States in December 2017 for primary open angle glaucoma and ocular hypertension as a second line therapy with only once daily administration.3 It is the first glaucoma drug of its kind, acting specifically on the trabecular meshwork as one of its three proposed mechanisms of action. The other mechanisms of action include norepinephrine transport (NET) inhibition and reduced episcleral venous resistance, the former resulting in ciliary body vasoconstriction causing reduced aqueous production and the latter allowing for increased aqueous outflow.4
Case 1
An 81-year-old African-American female presented for her quarterly glaucoma re-evaluation. She currently has pseudo exfoliative glaucoma (PXG), mild in the right eye, and severe PXG in the left eye. Currently treated with Travatan z qhs OU and Cosopt BID OU. Health history is remarkable for elevated lipids controlled with Lipitor. She had a previously documented allergy to brimonidine. Ocular history is remarkable for failed selective laser trabeculoplasty (SLT) OU in 2009, phacoemulsification/Mitomycin C trabeculectomy OU 2013 and Baerveldt tube shunt implantation OU 2017.
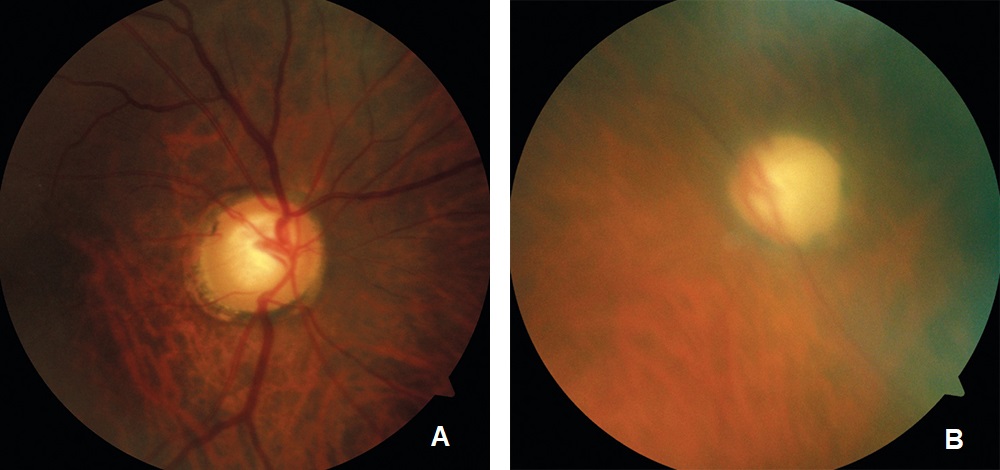
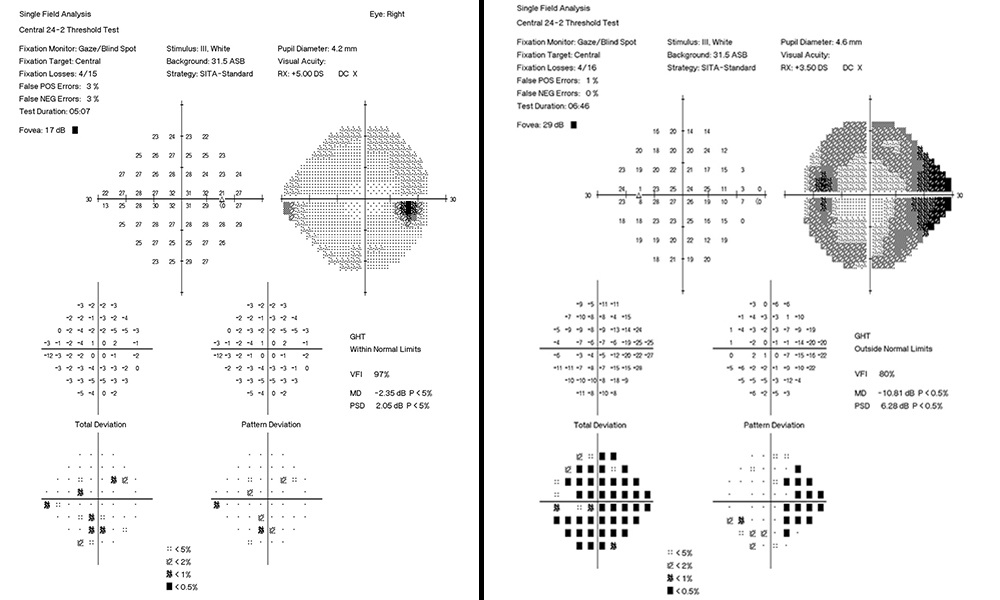
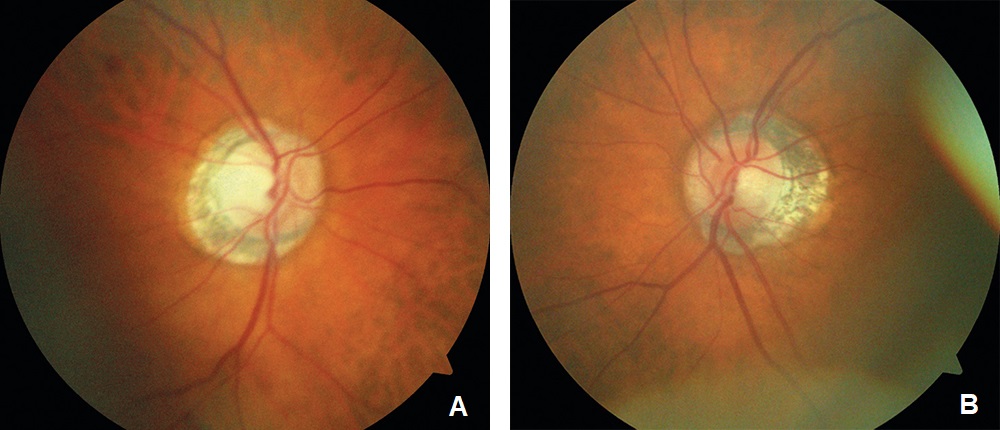
Entering visual acuity was 20/20- OD and Hand Motion OS. Decreased vision OS was due to advanced glaucomatous damage. She displayed an afferent pupillary defect OS and was unable to perform confrontation fields in the left eye. All other entrance testing was unremarkable. Slit lamp exam displayed bilateral superiorly placed MMC trabeculectomies with mild vascularization and no elevation. Well placed inferior Baerveldt tube shunts were observed inferonasally OU with no debris, blockage or iris touch of tube noted. Pseudoexfoliative material was noted bilaterally around the pupillary margins OU. Dilated fundus exam of the right eye displayed a parafoveal epiretinal membrane (ERM) in the macula and an intact neuroretinal rim with vertical elongation of the cup. The left eye displayed complete obliteration of the neuroretinal rim, macula clear. Bilaterally a ⅔ AV ratio of the retinal vessels was noted and no breaks or detachments noted in the periphery (Figure 1a and Figure 1b). The patient’s pachymetry readings (Reichert®, iPac Pachymeter) were 560 microns OD and 565 microns OS. Her untreated intraocular pressure (IOP) was 30mmHg OD and 32mmHg OS by Goldmann Applanation Tonometry (GAT). She presented at this visit with an IOP of 16 mmHg OD and 14 mmHg OS. Her target IOP for the right eye was low teens and target IOP for the left eye was comfortable.
The patient had not been consistently reaching a target IOP of low teens OD at the last two visits with reported proper compliance and drop instillation technique in-office with artificial tears. Rhopressa qhs OU was initiated and patient scheduled to return in two months for re-evaluation. Upon follow-up the patient presented with IOP by GAT measured at 11mmHg OD and 9mmHg OS, achieving a target IOP. All other findings were consistent with previous examination (Figure 2a and Figure 2b, Figure 3). On the next two subsequent visits the patient averaged an IOP in the right eye of 10 mmHg (9 mmHg, 10 mmHg), successfully maintaining her target IOP. This case focuses on an aggressive form of glaucoma in a monocular patient and the need for tight control of IOP. She has also previously endured invasive, incisional glaucoma surgery with few surgical options left. Rhopressa (0.02% netasurdil ophthalmic solution) allows for a daily administration schedule while achieving target IOP to further preserve vision. We initially dosed Rhopressa QAM to balance multiple drop instillation given our patients were already on 2 or 3 medications. For those highly sensitive to the hyperemia, we more recently have switched to QHS to reduce hyperemia during the day.
Case 2
A 67-year-old African American female presented for quarterly glaucoma re-evaluation. She is a normotensive glaucoma (NTG) patient staged at moderate OD and mild OS. Current glaucoma treatment includes Travatan Z qhs and Simbrinza BID OU. She received a neuro-eye examination yielding unremarkable blood work, head imaging and carotid ultrasound. She has an unremarkable medical history and denies migraines or obstructive sleep apnea. Her ocular history is remarkable for failed SLT in 2015 OU and denies any past blunt trauma or surgery to either eye. She has no known drug allergies.
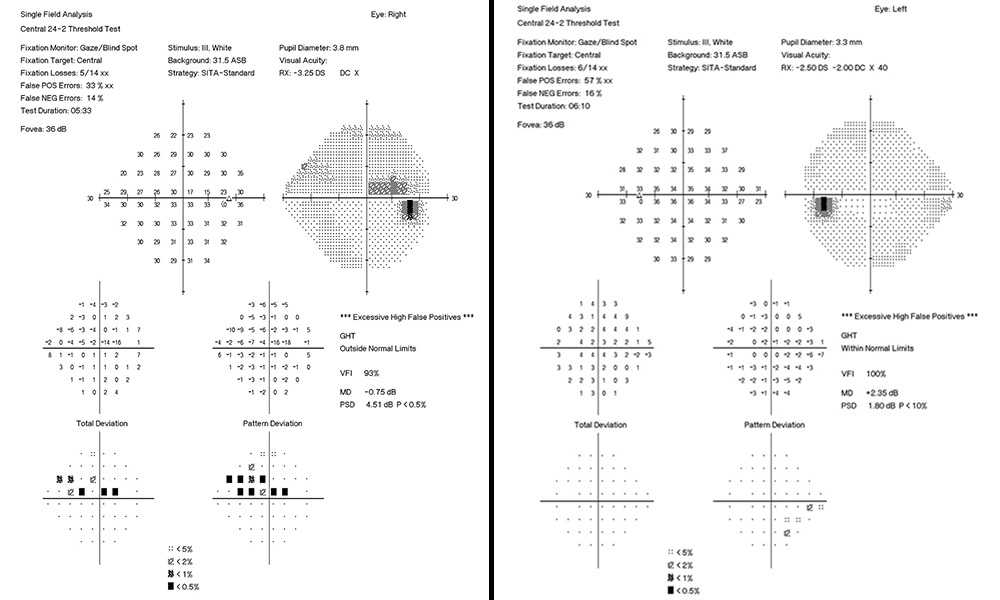
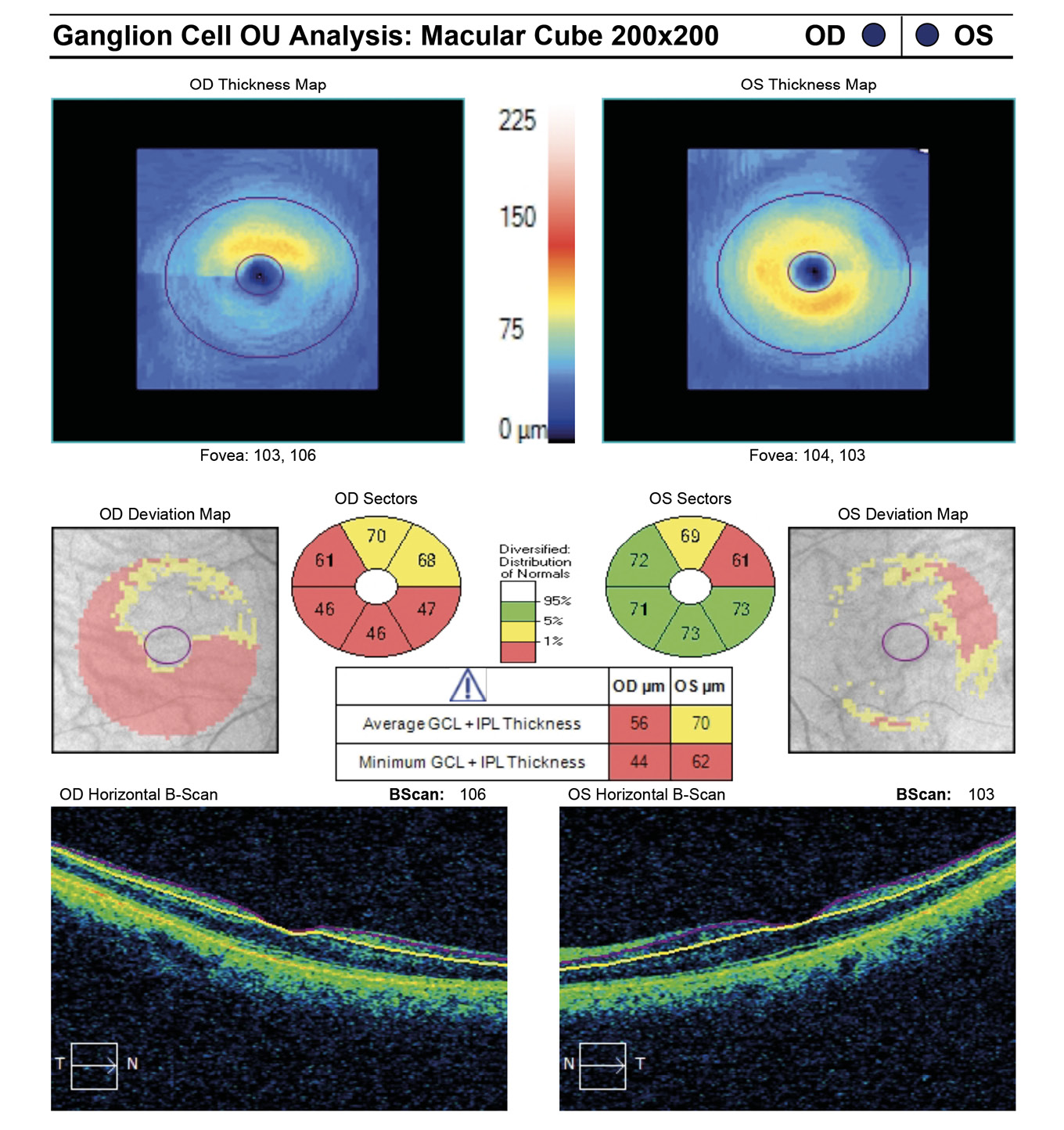
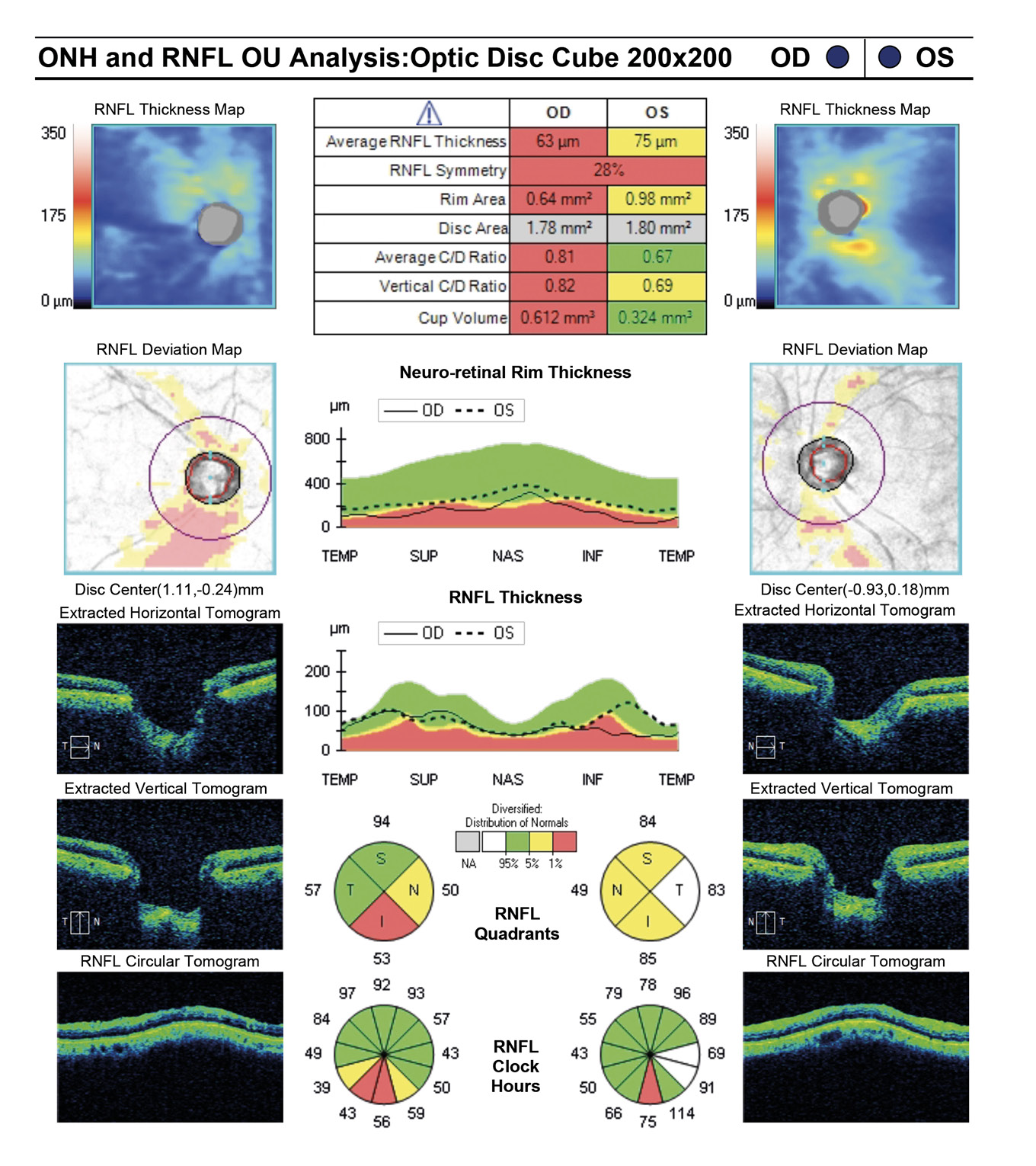
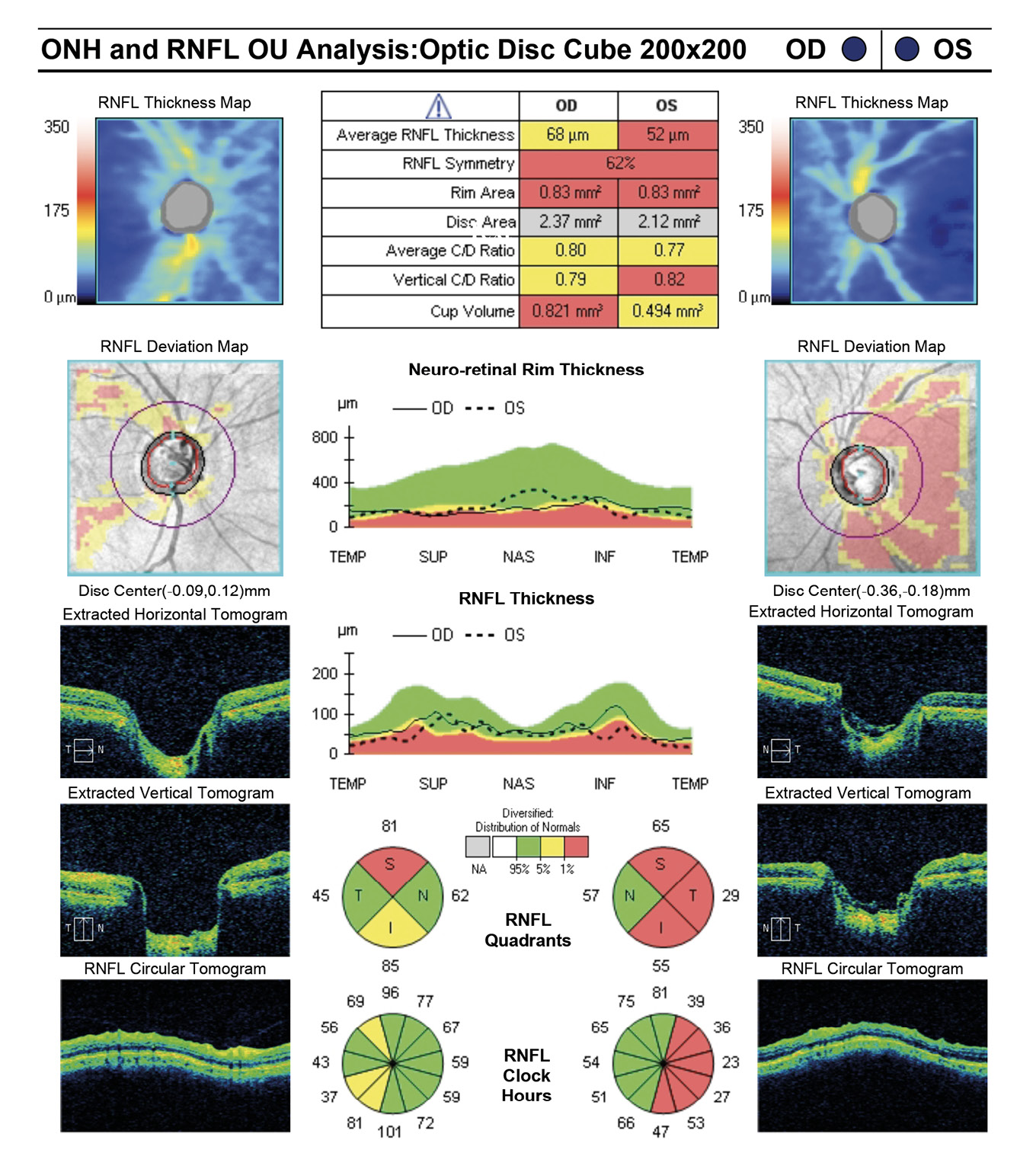
Entering visual acuity was found to be 20/20- OD & OS and all other entrance testing was unremarkable. Anterior segment slit lamp exam findings were unremarkable. IOP readings by Goldmann Applanation Tonometry (GAT) measured 16 mmHg OU. Pachymetry readings (Reichert®, iPac Pachymeter) were 506 microns OD and 507 microns OS. The patients untreated IOP at initial exam was 21 mmHg OD and 20 mHg OS. Upon first initiation of treatment with Travatan Z qhs OU, a target IOP of low teens or lower was established. Gonioscopy findings showed all angles open to ciliary body with a flat iris approach and no secondary factors to influence IOP OU. Remarkable fundus findings of the right eye include vertical elongation and inferotemporal neuroretinal rim thinning with marked beta-zone peripapillary atrophy. Remarkable fundus findings of the left eye reveal vertical elongation with an intact neuroretinal rim. CD ratios estimated to be 0.8/0.85 OD and 0.55/0.6 OS. (Figures 4a and 4b, Figure 5, Figure 6). Intraocular pressure was not at target pressure for the last three visits. Rhopressa QAM OU was initiated and patient was scheduled to return in two months for re-evaluation. Upon follow up the patient presented with reports of moderate conjunctival hyperemia in each eye that she stated as tolerable and painless. IOP measured by GAT were 12mmHg OU, achieving target IOP. All other exam findings were consistent with the previous visit. On follow-up evaluation IOP measured 9mmHg OD and 10mmHg OS. This case highlights the difficulty of lowering an IOP as close to 10 mmHg as possible in a normotensive (NTG) patient where laser procedures have already been attempted. Before Rhopressa, this patient would have few therapeutic options remaining and the next step would likely be incisional surgery. The option of a beta blocker in an NTG patient is controversial since it may affect perfusion pressure making treatment deleterious and was not considered as a viable option in this case.5,6
Case 3
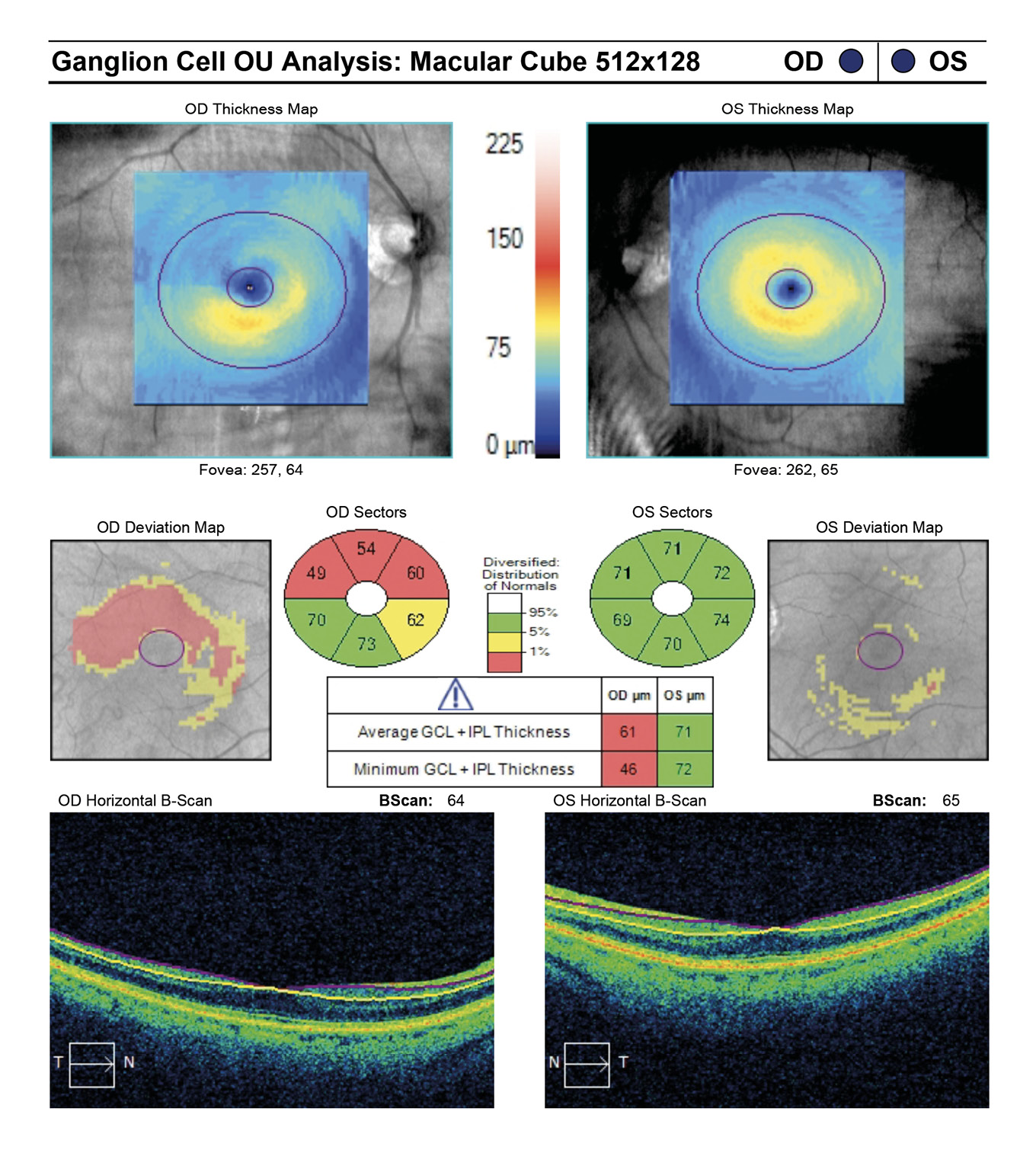
An 83-year-old Indian male presented for quarterly glaucoma re-evaluation. He has primary open angle glaucoma and is staged at severe OD and moderate OS. He is currently taking Travatan Z qhs OU. His systemic history is remarkable for prostate cancer in remission and denies diabetes mellitus or hypertension. His ocular history is remarkable for pseudophakia status/post YAG capsulotomy OU and denies any blunt injuries. He has no known drug allergies. Entering visual acuities are 20/20 OD and OS with unremarkable entrance testing. Slit lamp exam findings of the anterior segment were unremarkable. IOP measured by GAT were 18 mmHg OD and 15 mmHg. Historical pachymetry readings (Reichert®, iPac Pachymeter) measured 540 microns OD and 547 microns OS. Untreated IOP measurements were 25 mmHg OD and 26 mmHg OS. Gonioscopic findings displayed angles open to ciliary body with flat iris approach and no angle abnormalities. Fundus assessment displayed vertical elongation but an intact neuroretinal rim in each eye. Cup/disc ratios measured at 0.6/0.8 OU.
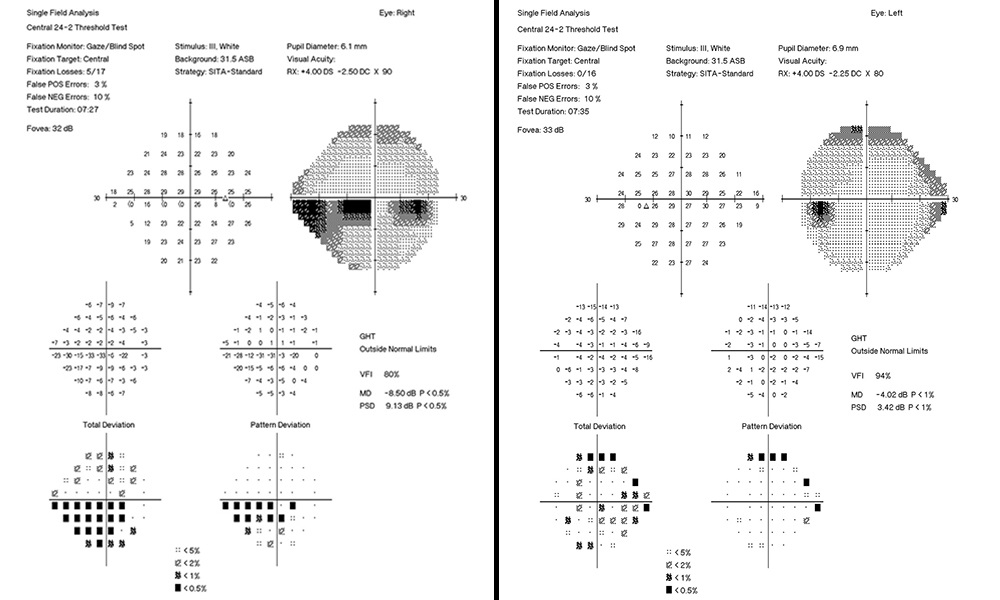
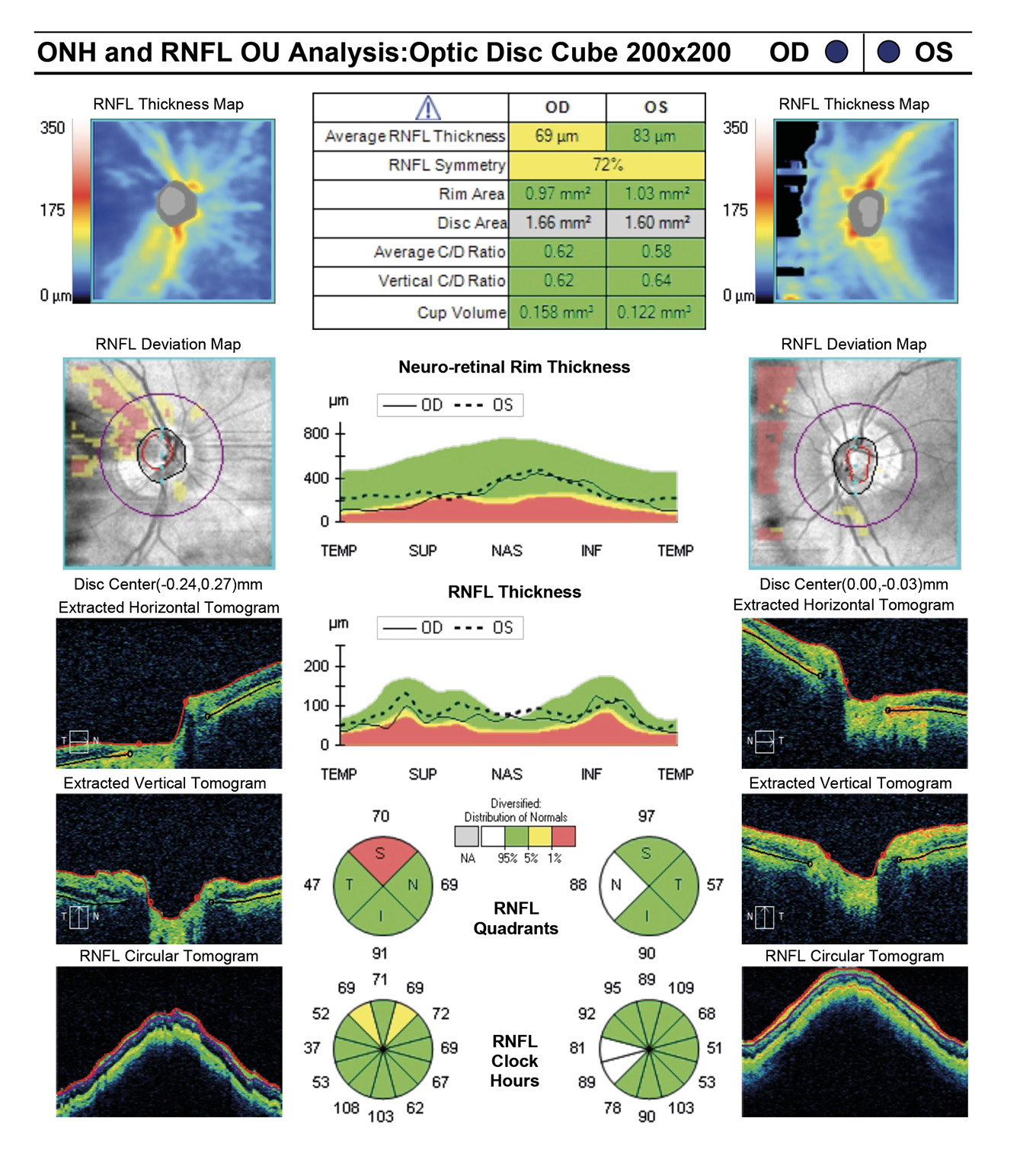
At initial visit a target IOP of low teens was established. The last several visits measured IOPs in the high teens. Rhopressa qhs OU was initiated and the patient was scheduled for re-evaluation in three months. At follow-up evaluation, IOP readings by GAT measured 13 mmHg OU, successfully achieving target IOP. All other previous exam findings remained consistent (Figure 7a and 7b, Figure 8, Figure 9, Figure 10).
Discussion
Primary open angle glaucoma patients possess a diseased trabecular meshwork (TM). Higher concentrations in the aqueous humor of glaucomatous eyes possess pro-fibrotic factors compared to normal eyes.4,7 Rho-associated protein kinase (ROCK) promotes cell fibrosis. Netasurdil’s ROCK inhibition has been proposed to possess anti-fibrotic activity. The drug works by inhibiting extracellular matrix production in the TM, decreasing expression of fibrosis-related proteins and reducing contraction of cells in both the TM and Schlemm’s canal.8,9 If a patient’s etiology of IOP elevation resides in the TM, Rhopressa can allow for further IOP lowering by essentially cleaning out the meshwork from past fibrosis and increase pore size. Additionally, Rhopressa was found in rabbits to inhibit norepinephrine transport (NET). NET inhibition works to block norepinephrine re-uptake, allowing norepinephrine to stay at the synapse in the ciliary processes resulting in reduced aqueous production.4,10, 11 If less blood flow occurs at the ciliary processes, less aqueous is produced, resulting in further reduction of IOP. Last, Rhopressa’s third proposed mechanism of action involves relaxation of vascular smooth muscle. The conjunctival blood vessels involved in episcleral venous resistance are likely relaxed. Vasodilation occurs resulting in the painless hyperemia experienced in many patients taking Rhopressa.4 Between the three mechanisms, we have seen an average IOP lowering of 2-4mmHg in the overall majority of our cases, consistent with what has been founder in large scale studies.12
Additionally, it has been shown that hyperemia is the most common side effect with lasting effects ranging from four to eight hours after instillation. Our recommendation to patients of nightly dosing allows for the worry of conjunctival hyperemia to be nullified, given the patient will be asleep during the most prevalent hours of unwanted conjunctival injection.4 Rhopressa addresses the issue of a diseased trabecular meshwork while also providing ways to facilitate improved aqueous outflow. Its most common side effect of hyperemia can be better tolerated by patients when taken at night given its short duration of action4. These cases display that Rhopressa can effectively further lower intraocular pressure given its unique mechanisms of action and serve as an appropriate adjunct in ones moderate to severely staged glaucoma cases.
Conclusion
In summary, ROCK inhibition relaxes the TM and increases Schlemm’s canal permeability by blocking formation of actin stress fibers and focal adhesions as well as promoting anti-fibrosis of the surrounding tissue. NET inhibition concomitantly results in reduced aqueous production via vasoconstriction of the ciliary processes blood vessels. Last, vasodilation of the episcleral veins reduces resistance of aqueous outflow, allowing us to achieve IOPs in the single digits on medical treatment alone.4 Rhopressa has been found at our clinic to be a viable adjunct therapy to lowering and sustaining a lowered IOP in moderate and advanced open-angle glaucoma. It is approved as a 2nd line of therapy, however given the drug is more difficult to get covered by insurance, it generally aligns with our patients as a third- or fourth-line drug. Our severe staged patients fit perfectly within this paradigm as they are already on what would be traditionally considered as maximal medical therapy (3-4 medications). When all other glaucoma medical therapy has been exhausted and traditionally surgical intervention would be considered next, Rhopressa is now more than ever being utilized as an alternative to incisional surgery. With its once daily administration, Rhopressa may improve compliance and provide an alternative treatment option for patients with refractory glaucoma on maximal medical therapy.13
Conflict of interest
The author declares that there is no conflict of interests regarding the methods and devices mentioned in the article.
