Risk factors for the development and progression of diabetic retinopathy
Purpose: Diabetes mellitus is a global disease in which a reduced insulin production or effect affects the blood sugar metabolism. The incidence of the illness is rising steadily. An elevated blood sugar level implies changes in the vascular walls and, in the long term, a reduced blood flow to some areas of the retina As a result, growth factors such as VEGF are overexpressed, which can lead to vitreous haemorrhage and traction-related retinal detachment via the formation of new blood vessels. The aim of this work is to review the literature to discuss the risk factors relevant for the development and progression of diabetic retinopathy.
Material and Methods: We carried out a differentiated literature review regarding the risk factors relevant to the development and/or progression of diabetic retinopathy based on a detailed description of the different forms of diabetes. The different risk factors were discussed and evaluated in the context of the different stages of diabetic retinopathy.
Results: The risk of retinal conditions increases with the duration of the underlying diabetic disease and a significantly higher blood pressure. In insulin-dependent type 1 diabetics, optimising the time in range can reduce the risk. In type 2 diabetics, whose disease is often associated with obesity and increased blood lipid levels, an increase in physical activity can be useful in addition to optimal blood sugar control. All factors that reduce blood circulation, such as nicotine consumption or an increase in intraocular pressure, are to be regarded as risk factors. The first visible ocular signs of diabetic retino pathy are outpouching of the vascular walls (microaneurysms), the number of which correlates with the risk of progression of the retinal disease.
Conclusion: It is crucial to understand the systemic and ocular risk factors to reduce the risk of developing or progressing diabetic retinopathy. A clear risk factor that can be reduced is nicotine. Interrupting an anti-VEGF therapy may lead to the progression of diabetic retinopathy.
Introduction
Diabetes mellitus is a chronic condition in which the body cannot produce sufficient amounts of the hormone insulin, or the hormone is not effective. Insulin is produced in the pancreas and controls the uptake of glucose into the body's cells, where it is used for energy production. A lack of insulin or of its effect on the cell leads to elevated blood sugar levels. In type 1 diabetes, which is usually diagnosed in childhood or adolescence, autoantibodies destroy the insulin-producing cells of the pancreas. Insulin must therefore be permanently administered from the time of diagnosis. It is generally assumed that this type of diabetes stems from a genetic predisposition, but viral infections and nutritional factors may also play a role. Less than 10% of diabetics worldwide suffer from type 1 diabetes.
In type 2 diabetes mellitus, which usually only occurs in adulthood in conjunction with increased carbohydrate consumption and obesity, insulin is not sufficiently effective on the body's cells and cannot introduce glucose. This is referred to as insulin resistance. The lack of the effect of insulin initially leads to insulin overproduction, but, in the long term, may cause exhaustion of the pancreas with a reduction in insulin production, so that the administration of insulin is then also necessary. Various studies estimate that more than 90% of diabetics worldwide suffer from type 2 diabetes mellitus. In the year 2000, the WHO declared an epidemic spread of obesity leading to an increasing number of type 2 diabetics, describing one of the most important risk factors for the development of diabetic retinopathy.1
In addition to the types mentioned above, there exist less common forms of diabetes mellitus: If a pregnancy is complicated by increased blood pressure, obesity or lipid metabolism disorder, gestational diabetes can occur, which usually disappears with giving birth, but is associated with an increased risk for the later development of type 2 diabetes mellitus for mother and child.2
In rare cases, secondary diabetes mellitus with decreased insulin production in adults can develop as a result of pancreatic diseases, hormonal disorders or medication intake.
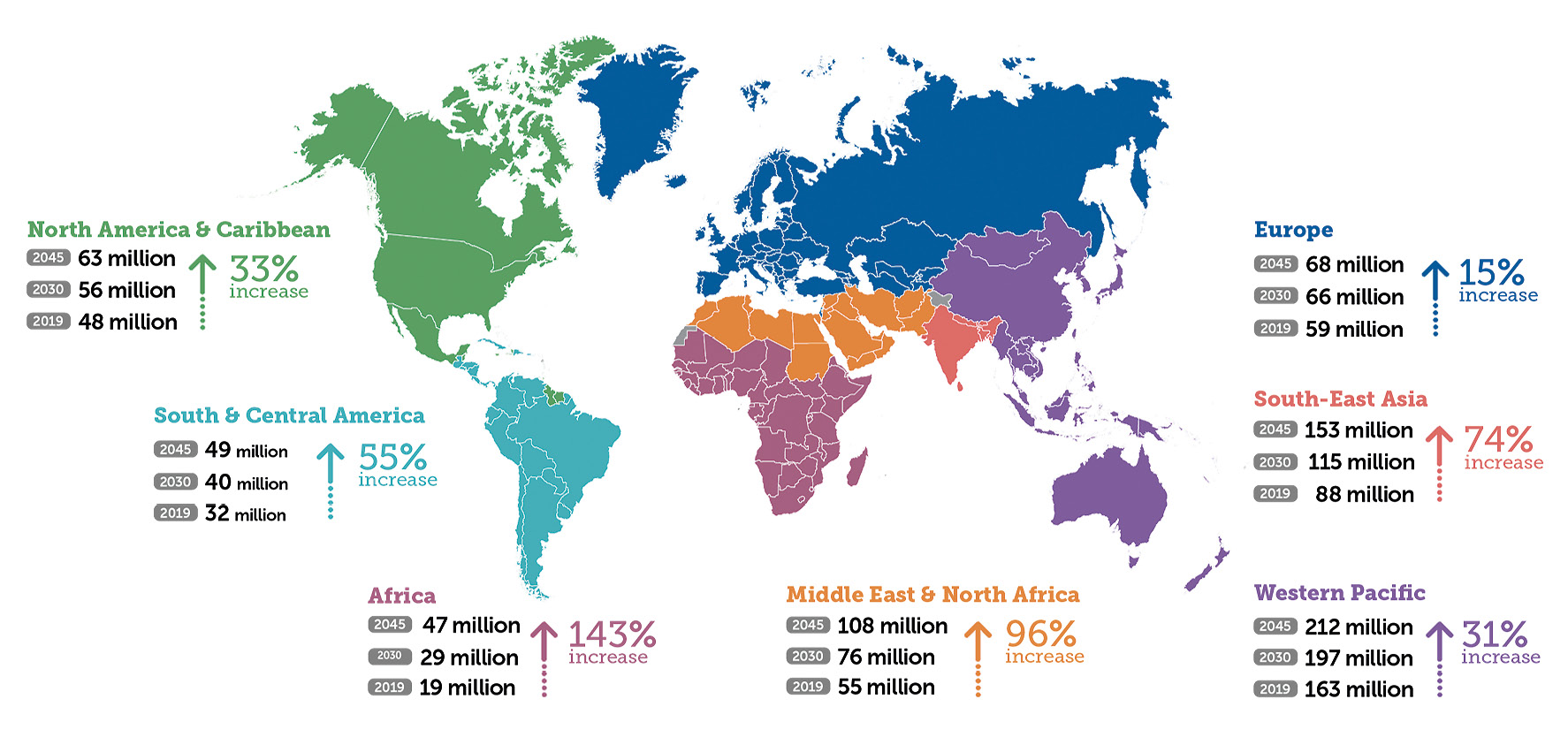
The International Diabetes Federation (IDF) estimates that, in 2019, 463 million people over the age of 20 suffered from diabetes mellitus worldwide; by 2045, an increase of 51% is expected, meaning that we will reach the mark of 700 million people.2 Since type 2 diabetes mellitus is related to growing economic prosperity, a sharp increase in prevalence is to be expected, especially in emerging countries: in Africa, where, according to the IDF, more than half of diabetics are currently unaware of their condition, an increase of 143% by 2045 is expected. Europe and the North American continent expect an increase of 15-31% over the same period (Fig. 1). Most of the world’s type 1 diabetics live in Europe and their number is also increasing, especially among children under 5 years of age.3,4
When the blood glucose level increases (hyperglycemia), biochemical and inflammatory mechanisms initially lead to a loss of the pericytes surrounding the blood vessels and surface changes in the erythrocytes, and subsequent occlusion of the blood vessels. Typical diabetic retinal changes develop together with neurodegenerative processes.5
Clinically, diabetic retinopathy goes through different stages: in the non-proliferative early stage, the vessel walls bulge (aneurysms). These changes are initially barely visible and do not cause any vision loss. In the course of the disease, capillary vascular occlusions result in hypoxic retinal areas with a reduced blood supply, in which growth factors such as the vascular endothelial growth factor (VEGF) and angiopoietin are upregulated and lead to the formation of new blood vessels. These fragile blood vessels that grow into the vitreous humour from the retinal vessels (proliferations) may bleed. The accumulation of connective tissue cells creates contractile membranes, which can cause tension-related retinal detachment (traction ablation). In addition to these changes, bulging blood vessels (microaneurysms) and increased permeability of the vessel walls can lead to accumulations of fluid in the centre of the retina (focal or diffuse diabetic macular oedema), which threaten central visual acuity. All of these conditions, the disease of the peripheral retina, diabetic retinopathy in the narrower sense and the disease of the macula (diabetic macular oedema) can occur simultaneously or independently of one another.
A large international meta-analysis that evaluated 35 studies from 1990 to 2008 shows that one in three diabetics worldwide exhibits retinal changes and more than one in ten suffers from vision-threatening diabetic retinopathy.12 This means that diabetic retinal changes are the most common cause of vision loss in people at working age. Vascular changes also occur in other organs and tissues, such as the kidneys, the peripheral nerves, the cardiovascular system and the extremities; an adjustment of the systemic risk factors also leads to the avoidance of complications in these tissues. The prevention of diabetes-related retinal complications is therefore a global task of the highest importance, both for the affected individual and for society. A consideration of the risk factors for the development and progression of diabetic retinopathy can help to achieve this goal.
The most important risk factors for developing diabetic retinal disease are the duration of the diabetes and poor blood sugar control.
Type and duration of diabetes
The development of diabetic retinal disease depends on the type of diabetes, the duration of the diabetic metabolic condition and cardiovascular risk factors. The Wisconsin Epidemiological Study of Diabetic Retinopathy (WESDR) published data on the frequency of diabetic retinal changes in over 1,300 type 2 diabetics in 1984. After almost 5 years of diabetes, 29% of the patients showed signs of diabetic retinopathy, after 15 or more years of disease, diabetic retinal changes were visible in 78% of the examined test subjects. The prevalence of severe diabetic retinopathy with neovascularisation increased from 2% to 16% over the same period.
The almost 1,000 patients with type 1 diabetes mellitus were affected much more frequently: in their case, the prevalence of diabetic retinal changes increased even more with increasing duration of the disease: from 17% (after less than 5 years) to 97.5% (after 15 or more years). After more than 35 years of diabetes, the prevalence of severe retinopathy with neovascularisation was 67%.6 Over time, the risk of developing retinal complications has been reduced somewhat thanks to earlier detection of the underlying disease and more effective blood sugar control. Nevertheless, the large cross-sectional study from the German-speaking area, the German diabetes documentation system from 2011, shows that, out of almost 9,000 type 1 diabetics, after 40 years of illness, 84% presented retinal changes and more than 50% had severe retinal changes with proliferation.7
Also for type 2 diabetics, in whom the onset of the disease cannot be defined as clearly as in younger patients, the duration of diabetes mellitus and the quality of the blood sugar control are still one of the most important risk factors for the development and progression of diabetic retinopathy.8 The German Gutenberg study with data from 15,000 test subjects supports this statement. Another startling result of this study was that 28% of the approximately 1,000 diabetics within the test group were unaware of their diagnosis. They were only diagnosed with diabetes mellitus through the typical diabetic retinal changes.9
Blood glucose levels
In addition to the duration of the illness, the extent of the hyperglycaemia is another risk factor for the development and progression of diabetic retinopathy. Ideally, insulin therapy should bring the blood sugar levels back to the normal range in insulin-dependent patients. As early as 1993, the Diabetes Control and Complications Trail showed that an intensified blood sugar control, which lowers the blood sugar levels with the help of an insulin pump or at least 3 insulin doses per day to almost physiological values, can lower the risk of developing diabetic retinopathy within 6.5 years by 76% and the progression of an existing diabetic retinal disease compared to conventional blood glucose control with only 1 to 2 insulin takings per day by 54%.10
The punctual blood sugar measurements using a lancet (sugar control with a drop of blood) are needed to determine the required amount of insulin. The quality of the blood sugar control over several weeks is determined by measuring the glycated haemoglobin value (HbA1c). A German-Austrian study with almost 36,000 type 1 diabetics showed that fluctuating glycated haemoglobin (HbA1c) levels are the most important independent risk factor for the development and progression of diabetic retinopathy.11 All related studies confirm this observation also for type 2 diabetics.12,13,14 However, the HbA1c value is to be regarded as an average value in which both hyperglycaemia and hypoglycaemia can remain undetected. Strong fluctuations in blood sugar levels are problematic, however, since low blood sugar levels (hypoglycaemia) also contribute to the development of vascular changes, especially in type 1 diabetics.
With the technical development of blood glucose sensors15, it is now possible to measure blood sugar levels almost continuously and, thus, to monitor compliance with a target blood sugar value over time.16 Optimising this "time in range" leads to a reduction in the prevalence of all stages of diabetic retinopathy in patients.17 Continuous subcutaneous insulin administration can also improve the "time in range" and reduce the risk of secondary diseases.18
However, consistent blood sugar control cannot always prevent retinopathy from progressing. In about 5% of patients, especially after a long history of diabetic metabolism, there may be an increase in diabetic retinal changes within 12 months despite good blood sugar control. This phenomenon is known as "early worsening" and is believed to be caused by a genetic predisposition.19 This phenomenon underlines the urgent need for close interdisciplinary cooperation between ophthalmological and internal medicine. A Dutch study shows that in type 1 diabetics, in addition to fluctuations in HbA1c and the duration of diabetes, a higher patient age at initial diagnosis is another risk factor for the development of diabetic retinopathy. Advanced natural vascular aging and increased inflammatory reactions are discussed as causes for this13, but possibly older age at the time of initial diagnosis also means a longer untreated duration of the disease.
Blood pressure
In addition to regulating blood sugar levels, all other cardiovascular risk factors must also be minimised in order to prevent or delay the development of diabetic retinal changes. As shown in almost all related studies, arterial high blood pressure is one of the main risk factors for the development of diabetic retinopathy in addition to increased blood sugar.20,12,22,14 For diabetics who also have arterial hypertension, the risk of developing diabetic retinopathy increases by a factor of 1.7. Biochemical processes in the vascular endothelium can contribute to this.6 A study on increased retinal venous pressure also provides information on this. 21
Various studies such as the ACCORD EYE study23 and another international meta-analysis24 show that the risk of developing diabetic retinopathy may be reduced by adequately lowering blood pressure, but a direct correlation cannot be established. A target blood pressure of 140/90 seems to be sufficient; a further lowering seems to not have any additional positive effect on the retina22. This may indicate a minimum blood pressure value that is necessary for the perfusion of previously damaged blood vessels.
Since a British study also shows that less than 40% of patients achieve these target blood pressure values despite medication being prescribed25, early and interdisciplinary care of diabetes patients is of great importance.
As with blood sugar control, an optimised blood pressure can be facilitated by weight loss and physical activity26, which also have a positive influence on the harmful blood lipid levels.
Physical activity and body weight
Physical activity facilitates weight loss and helps control blood pressure and blood sugar levels while reducing harmful blood lipid levels.26 Studies show that the urban population has a higher risk of developing diabetic retinopathy than the rural population, this difference being attributed to more physical activity and less fast food consumption of the latter.2 A monocentric Japanese study was able to show that type 2 diabetics with increased physical activity present a lower incidence of diabetic retinopathy.27
A connection between increased body weight and the development or severity of diabetic retinopathy has been investigated in various studies with inconsistent results: Ethnicity and thus genetic factors, but possibly also differences in diet, seem to play a role. Interestingly, the presence of increased abdominal fat must be assessed as a risk factor for the development of diabetic retinopathy8, although this is more important for type 2 diabetics. For these patients, the German Gutenberg study shows a clear connection between increased body weight and the risk of severe vision-threatening diabetic retinopathy.9
Blood lipid levels
Elevated blood lipid levels, especially low-density lipoproteins (LDL) and cholesterol, lead to the formation of hard exudates and are associated with an increased risk of vision loss in diabetics. Furthermore, the probability of developing diabetic macular oedema with hard exudates that reduce vision increases22,28. According to an Indian study, the risk of developing diabetic retinopathy is also higher with increased serum levels of the harmful LDL lipid values, while higher HDL lipid values have a protective effect29, which also applies to atherosclerosis.
Nicotine
Nicotine consumption leads to a narrowing of the blood vessels and is also one of the risk factors for the development of diabetic retinal changes.9,12,30 Changes in the retinal structure after nicotine administration have been demonstrated in an experimental study.31
Which local factors in the eye increase the risk of developing or progressing diabetic retinopathy?
Microaneurysms and retinal ischemia
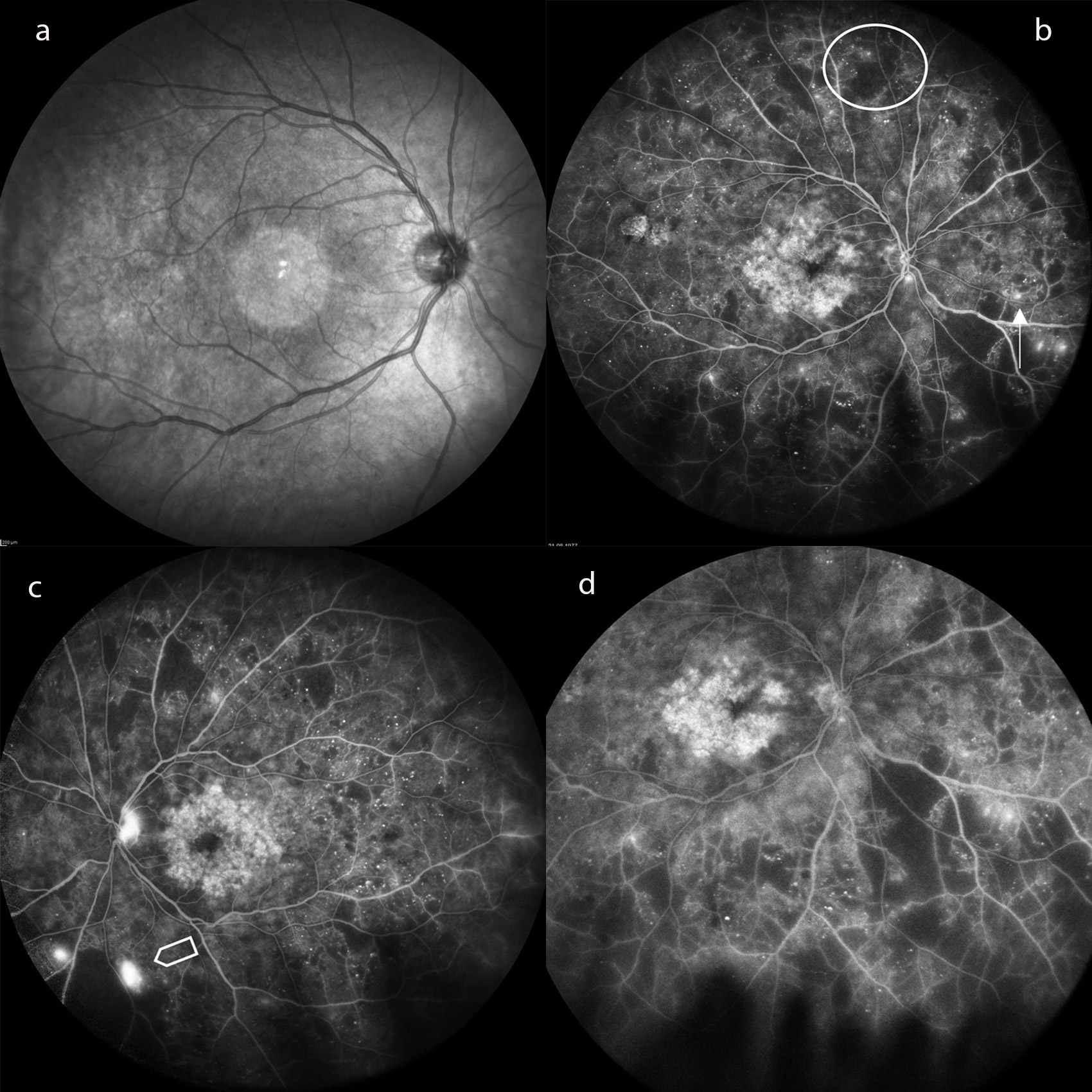
The number of microaneurysms in the retinal vessels is an indicator of the development of diabetic retinopathy, meaning that these can be assessed as local risk factors. They serve as a funduscopic screening instrument. Ischemic retinal areas that are not perfused with blood mark the transition to a proliferative retinopathy that threatens vision and can be easily visualised in fluorescence angiography (Fig. 2). For this purpose, ultra-wide-angle and wide-angle devices are superior to the conventional 30–50-degree display.32
Primary open-angle glaucoma
A new study shows that type 2 diabetics with primary open-angle glaucoma have a three-fold increased risk of developing diabetic retinopathy33 and highlights older data showing that increased intraocular pressure decreases vascular perfusion and increases the risk of diabetic retinopathy progression.34 The optimal adjustment of ocular pressure is therefore particularly important for diabetics.
Cataract surgery
The diabetic metabolism favours the development of cataracts. Whether cataract surgery with phacoemulsification and an intraocular posterior chamber lens carries the risk of progression of diabetic retinopathy has been investigated since the introduction of modern lens surgery.35 A British study showed that cataract surgery without complications leads to the disruption of the blood-retinal barrier and an increase in the aqueous humour concentration of VEGF and other vasoproliferative factors.36 Another study, on the other hand, found no influence of lens surgery on pre-existing diabetic retinal disease.37
However, since a good view of the fundus is essential for the early detection of diabetic retinal changes, a necessary cataract operation should not be delayed. In order to be able to perform laser coagulation as a therapy for early proliferative retinopathy, lens surgery may be indicated from a retinological point of view.
anti-VEGF therapy
Intravitreal therapy with anti-VEGF antibodies has been used since its approval based on the RESTORE study for the treatment of certain forms of diabetic macular oedema.38 These antibody compounds inhibit the development of diabetic retinopathy.39 However, studies show that stopping such therapy can produce the opposite effect in about 30% of patients leading to a short-term progression of diabetic retinal changes.40 Since diabetics often suffer from cardiovascular diseases, a suspension of the follow-up check-up is to be expected in about a third of the patients during long-term therapy. If anti-VEGF therapy is used as an alternative to laser therapy for the treatment of proliferative diabetic retinopathy, for which it has been approved since 2019, interrupting the therapy can become a risk factor for the progression of retinal changes. The German Ophthalmological Society (DOG) and the Retinological Society therefore recommend regular checks and to follow strict indications (www.dog.org).
Conclusions
It is important to educate patients at the onset of diabetes about the factors that lead to the development and progression of retinal diabetic disease and to help them lead a healthy lifestyle. Out of all risk factors, the most controllable one is nicotine. In order to avoid diabetic retinopathy, type 2 diabetics should also focus on increasing physical activity and thus reducing body weight, both of which lead, in most cases, to a reduction in blood sugar levels. For type 1 diabetics, optimal blood sugar control through modern technical equipment for blood glucose measurement and precise insulin administration is essential. Interdisciplinary internal and ophthalmological care for the patient is of crucial importance in order to avoid or to detect early retinal changes.
Conflict of interest
The author has no conflict of interest with regard to the methods and devices mentioned in the article.
Guideline of the DDG and DGGG (S3 Level, AWMF Registry Number 057/008, February 2018). Geburtshilfe Frauenheilkd., 78, 1219-1231.
Type 1 Diabetes Mellitus: Systematic Literature Review and Narrative Synthesis. JMIR Diabetes, 6, e20973.
