Visual rehabilitation with a scleral lens in an atypically flat and crosslinked keratoglobus cornea
Purpose: The case report outlines the fitting of scleral lenses to address visual impairment with spectacle correction in a patient exhibiting bilateral atypically flat keratoglobus characterised by severe corneal irregularity which occurred three weeks subsequent to unilateral corneal crosslinking (CXL) surgery, aimed at visual rehabilitation.
Material and Methods: Scleral lenses were fitted for a 15-year-old Asian patient who had no previous experience with contact lenses. This individual presented with an atypical, highly flattened, and irregular keratoglobus. The fitting process involved utilising topography parameters, diagnostic lenses, and anterior segment OCT for a comprehensive assessment.
Results: With the successful fitting of scleral lenses, an ex-cellent level of physiological tolerability has been attained, allowing for a daily wearing time of 15 hours consistently over a period of 12 months. Visual acuity has shown significant improvement, with an increase from the spectacle acuity of visual acuity 20/25 p and 20/32 to visual acuity 20/16 and 20/20 with the scleral lenses. As a result, the patient now experiences an enhanced quality of life in both educational pursuits and leisure activities.
Conclusion: In cases of both central and peripheral corneal irregularities, as well as complex corneal situations characterised by extreme flatness, scleral lenses emerge as a well-established solution for visual rehabilitation, surpassing the optical and geometric limitations of other contact lens types. Post corneal crosslinking (CXL), they can be fitted and worn earlier in comparison to corneal rigid contact lenses (RCL) owing to their corneal vaulting. Careful diagnostic lens fitting, guided by Anterior Segment OCT technology, and regular follow-up examinations contribute to the selection of an appropriate lens design, ensuring long-term physiological tolerance of scleral lens wear. This approach can significantly improve the daily lives of individuals who would otherwise face visual impairment challenges.
Introduction
Scleral lenses have gained widespread acceptance and are now extensively employed for visual rehabilitation in cases of irregular corneal astigmatism.1 They offer distinct advantages over corneal rigid contact lenses (RCL), particularly for challenging ocular surfaces, thanks to their corneal vaulting. Common applications include conditions such as Keratoconus, Pellucid Marginal Degeneration, Keratoplasty, and irregular astigmatism resulting from traumatic or infectious scarring.2–5 Keratoglobus is also recurrently cited as an indication, though literature documenting cases fitted with scleral lenses remains limited.6,7 To the best of the author‘s knowledge, there is no reported case of keratoglobus following corneal crosslinking and subsequent scleral lens fitting.
This case report details the fitting of scleral lenses on an atypically flat keratoglobus post-corneal crosslinking, utilising fitting lenses and Anterior Segment-OCT (AS-OCT) to precisely refine the fit on the ocular surface.
Material and Methods
A 15-year-old male Asian patient was referred to the specialty lens clinic by a cornea specialist in July 2022 for the visual rehabilitation of irregular corneal astigmatism. The initial diagnosis in 2017 at the eye clinic identified posterior amorphous corneal dystrophy due to posterior corneal opacities, with neovascularisation presenting as an atypical feature for the diagnosis. Over the subsequent five years, the local ophthalmologist monitored the patient, referring him again to the cornea specialist in 2022 due to progressive thinning and peripheral neovascularisation.
After five years, Scheimpflug imaging revealed pro gressing irregular corneal thinning in both eyes, leading to the diagnosis of keratoglobus, which can be associated with stromal opacities near the endothelium. Corneal crosslinking (CXL) was performed on the left eye, which exhibited more advanced disease, one month after the diagnosis.
The patient visited the specialty lens clinic three weeks post-CXL for lens fitting and was prescribed topical dexamethasone 0.1% three times daily. His bestorrected visual acuity (BCVA) with his current spectacles was 20/25 in the right eye (OD) and Vis 20/32 p in the left eye (OS). His spherocylindrical prescription was OD +5.00 −1.00 75° and OS +1.50 −6.00 105°. He reported house dust and seasonal allergies. In his ocular health history, the patient mentioned wearing glasses since the age of nine and being diagnosed with an irregular corneal surface at age seven. Moreover, no additional concerns arose regarding the patient‘s personal and family medical history. He continues attending public school and enjoys playing soccer on his local team. His chief complaint was related to distance vision and the discomfort of heavy glasses while playing soccer.
Ocular findings
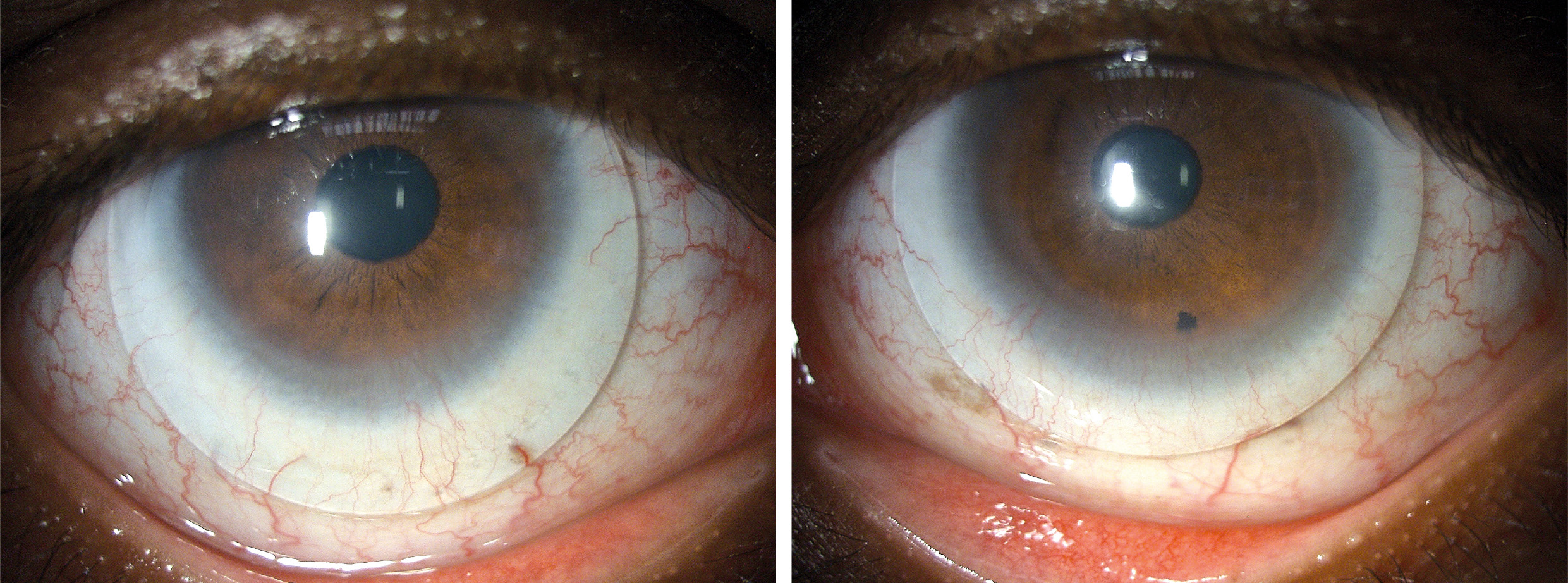
The slit lamp examination unveiled a general thinning of the cornea with localised extreme thinning, particularly pronounced in the supero-nasal quadrant near the limbus of the left eye. Circumferential pannus-like neovascularisation was observed and documented through digital imaging (Figure 1). Additionally, an exceptionally flat corneal shape was identified. Both eyes exhibited moderate hyperaemia in the conjunctiva and moderate limbal redness, as assessed by the BHVI Grading Scale (Brian Holden Vision Institute). Fluorescein staining was negative in the right eye and mildly positive in the left eye.
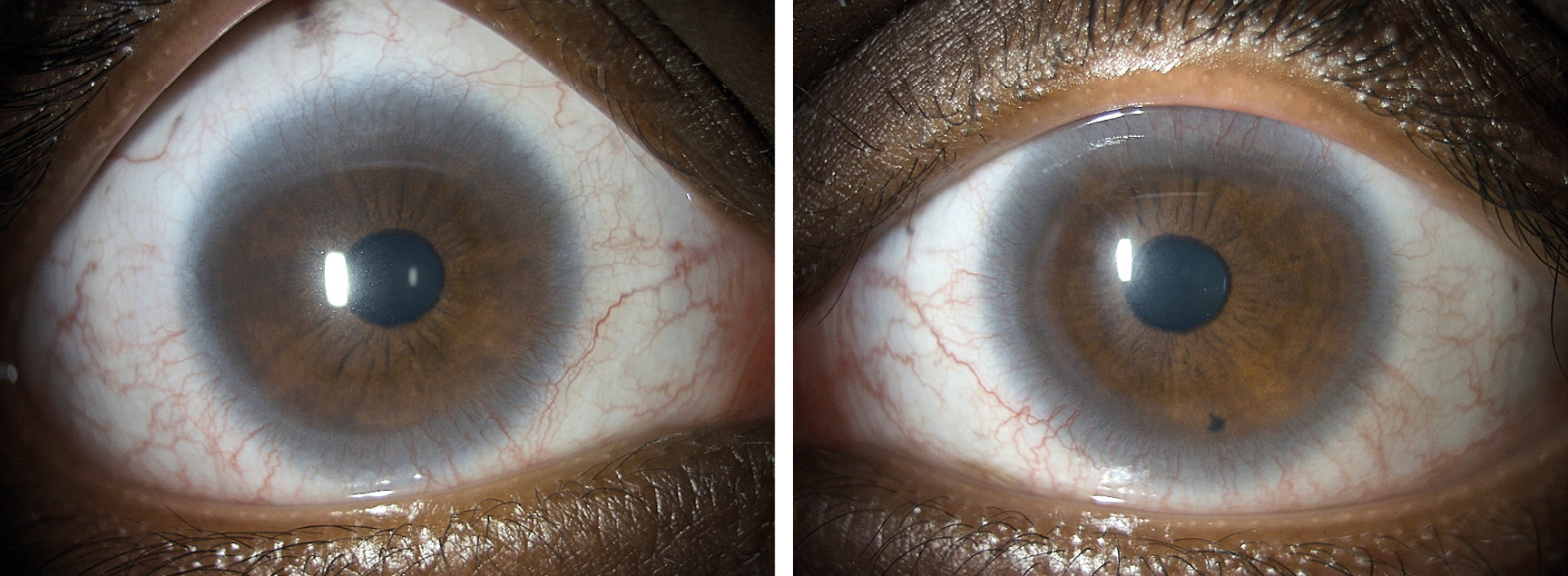
Corneal topography using the Oculus 5M (Oculus Germany) revealed highly irregular and flat corneal surfaces in both eyes. The corneal curvature in the right eye averaged 10.20 mm (32.8 D), while in the left eye, it measured 8.20 mm (41.7 D), with a flat meridian of 9.23 mm (36.6 D) (Figure 2)
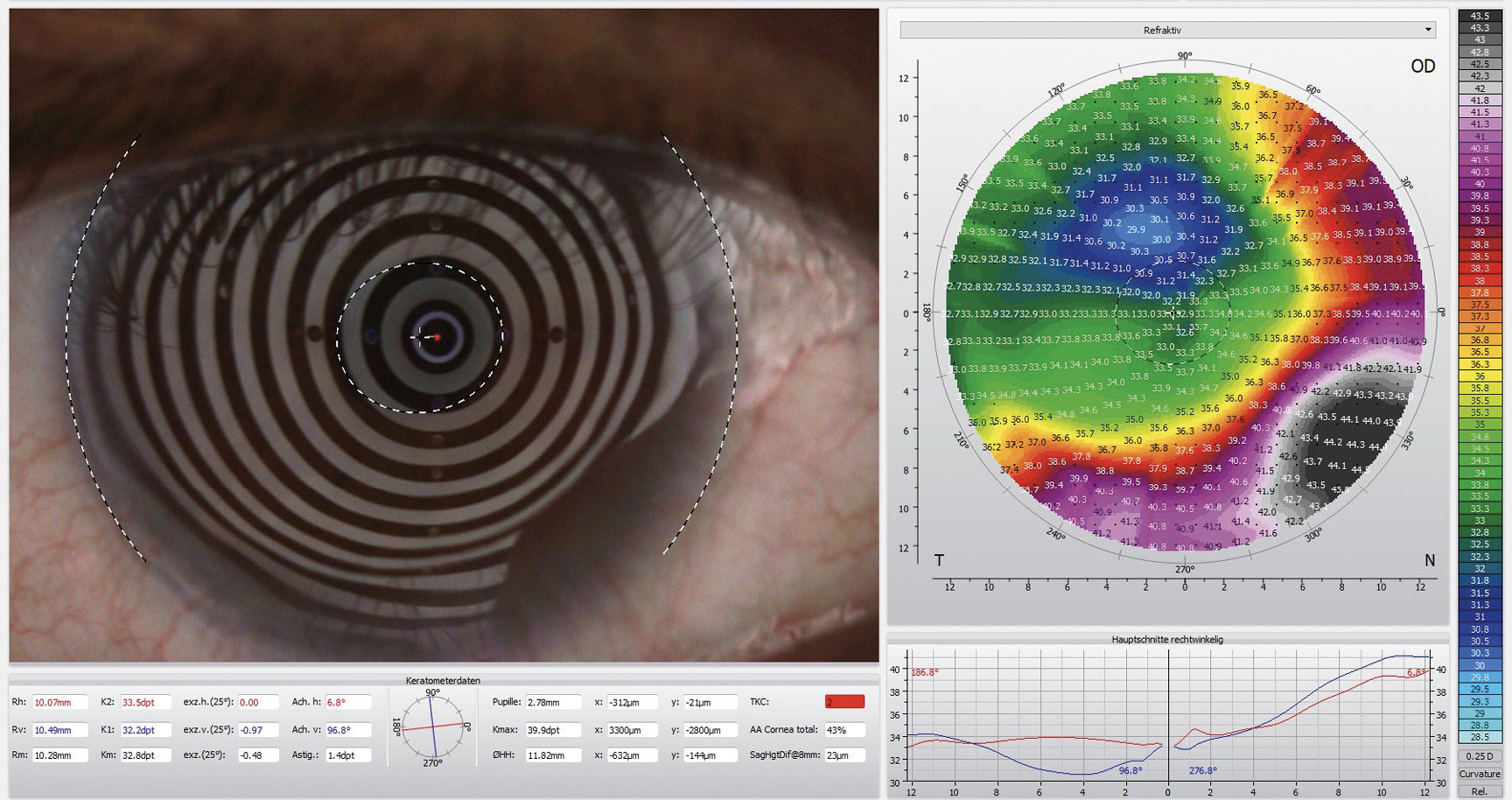
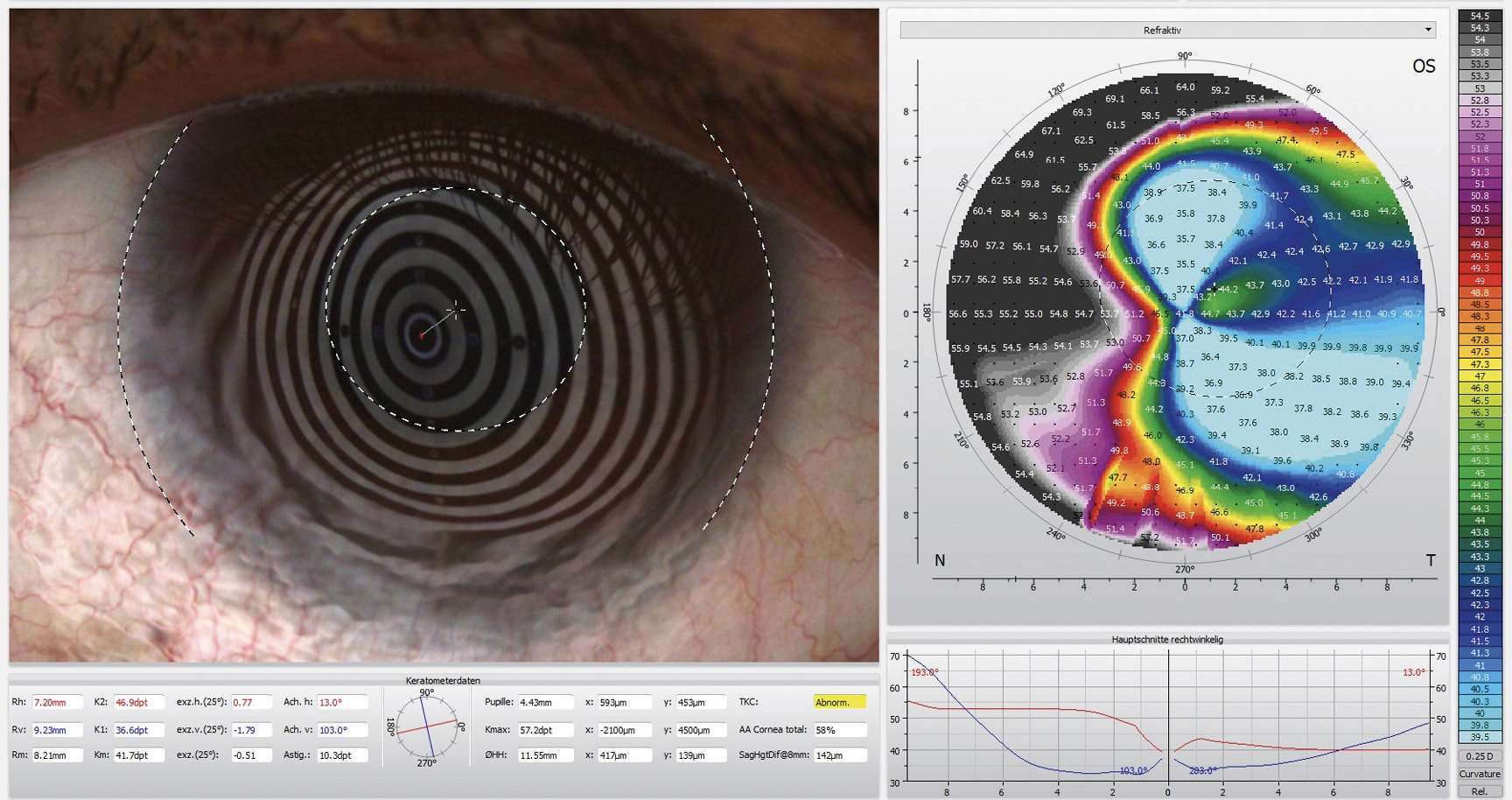
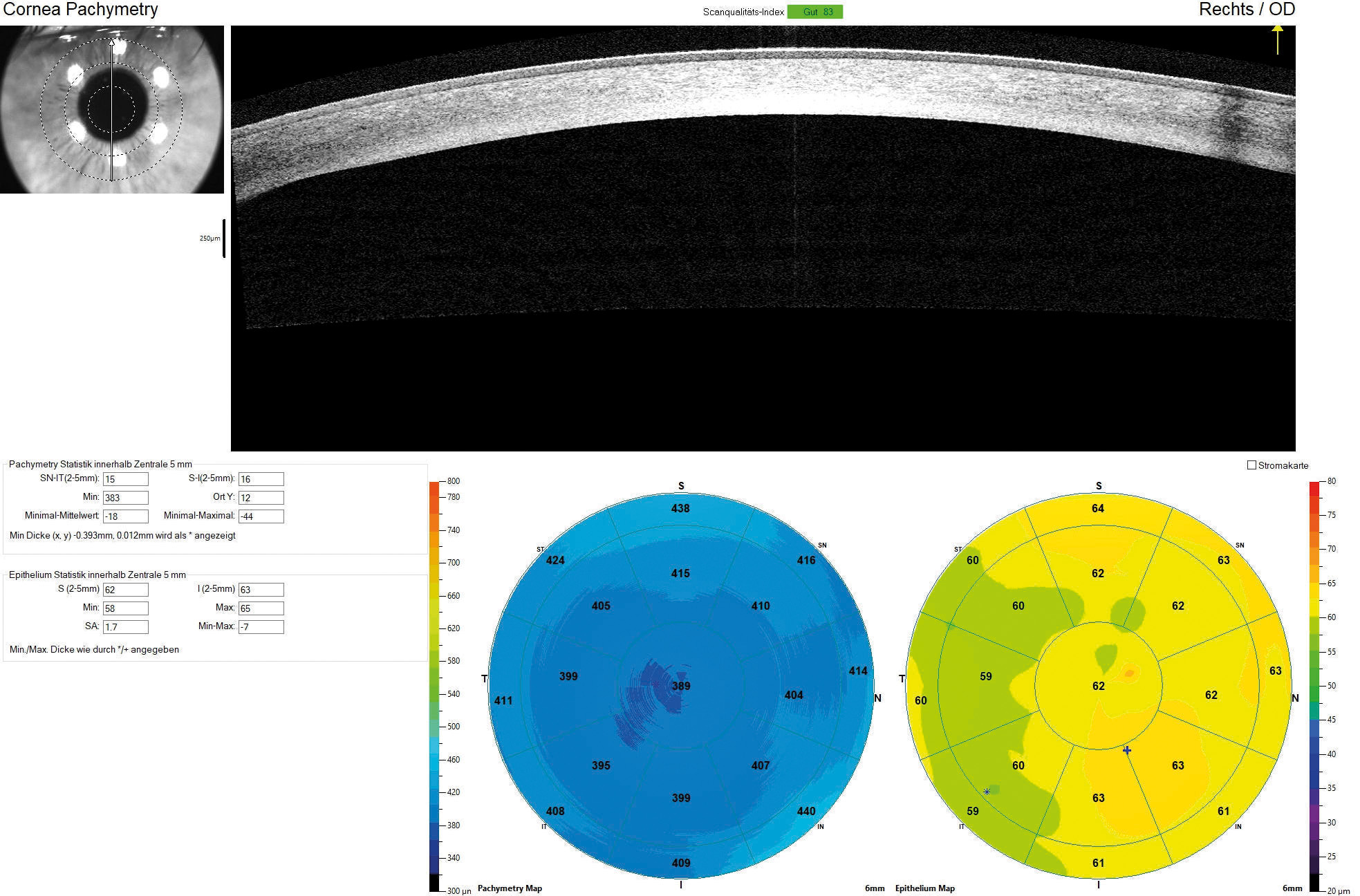
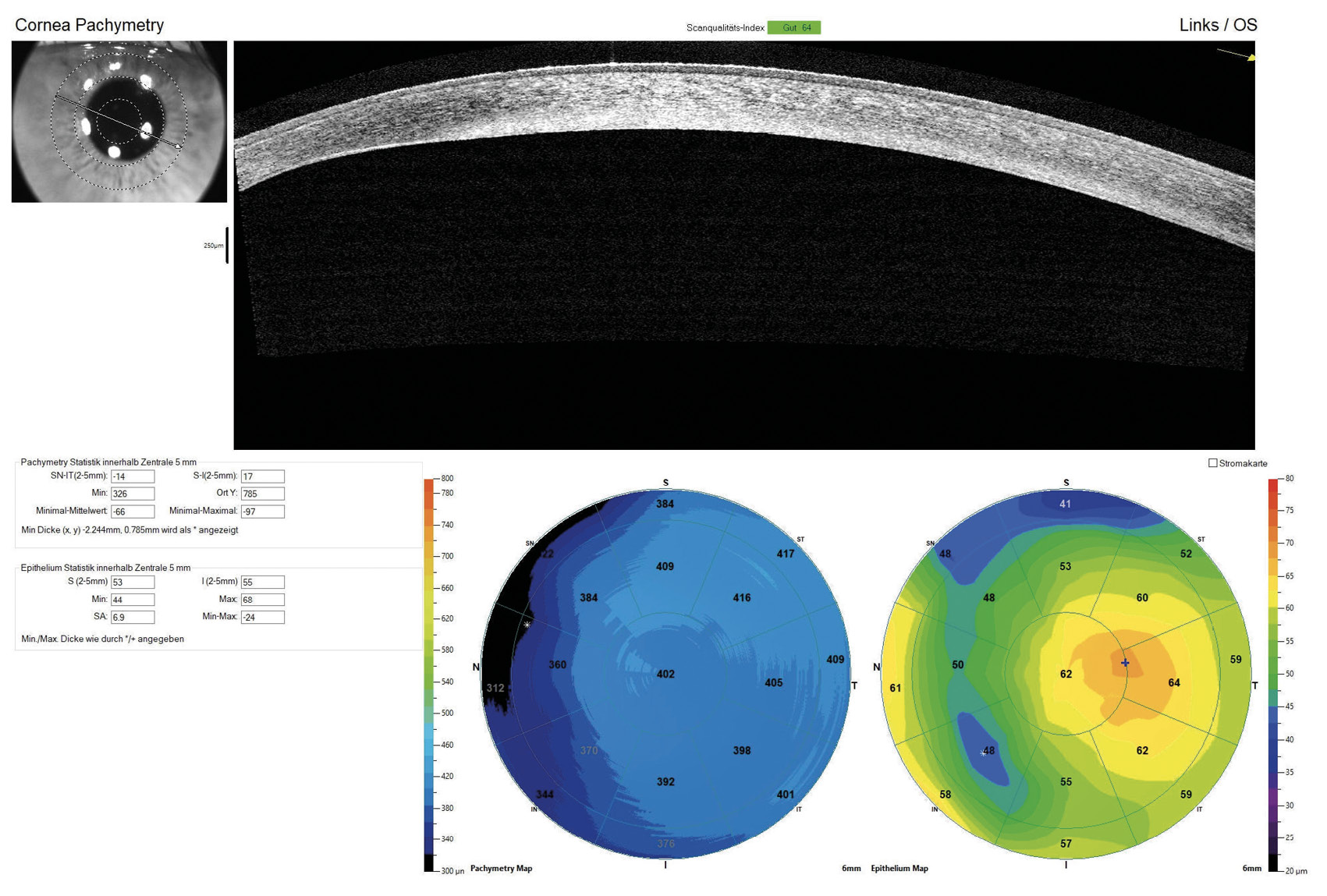
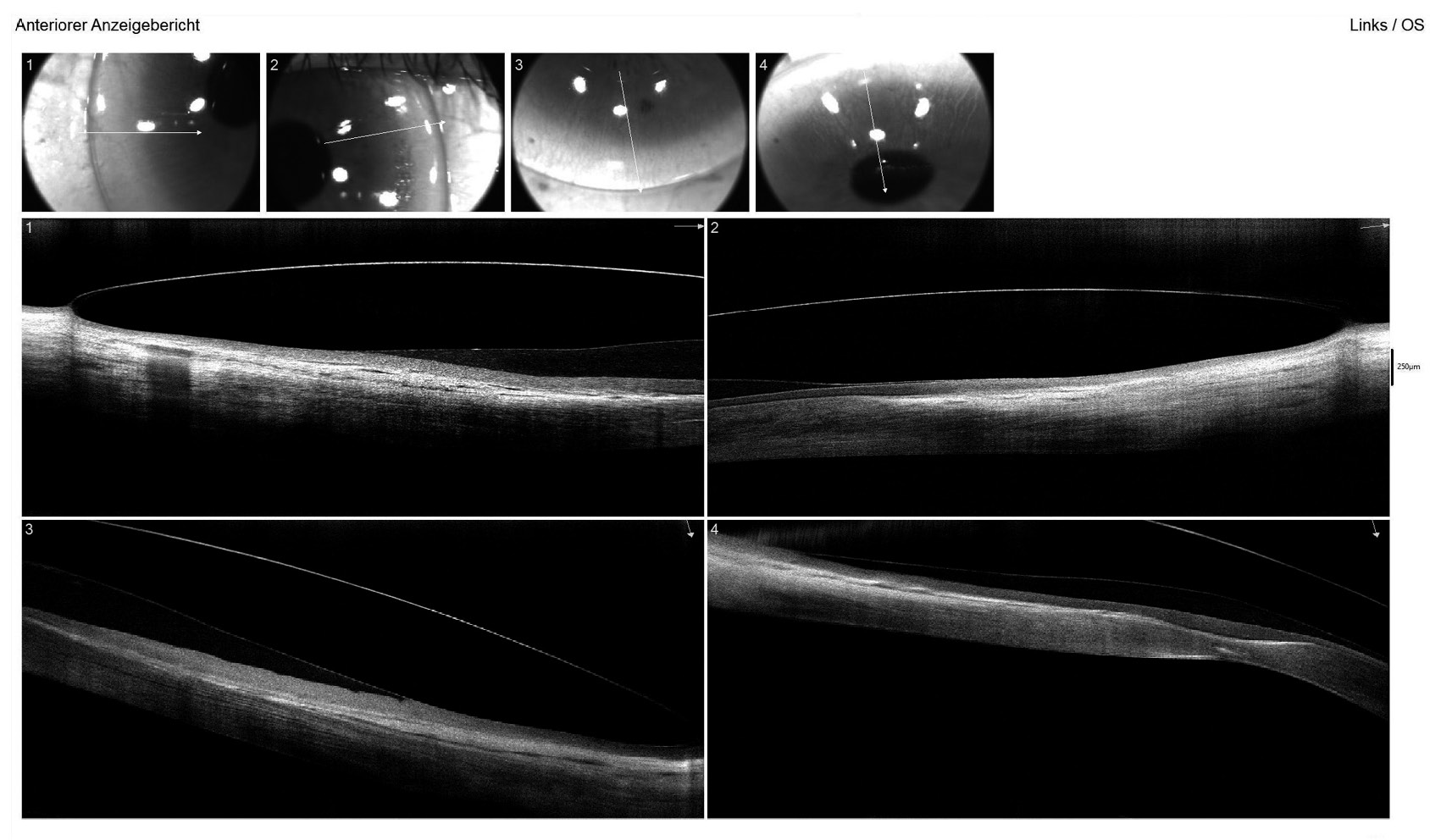
The horizontal visible iris diameter (HVID) was below average at 11.20 mm in both eyes. AS-OCT pachymetry (Optovue iVue OCT) disclosed a central corneal thickness (CCT) of 389 μm in the right eye and 402 μm in the left eye (Figure 3). The supero-nasal quadrant of the left eye exhibited a minimal stromal thickness of 170 μm near the limbus.
Given the flat ocular shape and irregular cornea properties, the fitting of contact lenses was anticipated to be challenging based on experience. The superior protrusion posed an additional fitting challenge, as it was expected that small diameter RCLs would decentre on the flat cornea.Large diameter RCLs necessitated complex geometries and were likely to touch with abrasions at the areas of superior ectasia. Fitting soft lenses was not considered as an option, as it was not expected to improve visual acuity compared to spectacles. As a result, a scleral lens fit was pursued to ensure optimal vision, lens centration, and clearance in the protrusion areas, a decision supported by the cornea specialist.
Fitting of scleral lenses
At the specialty lens clinic, scleral lens fitting was conducted utilising empirical corneal topography data, including base curve and horizontal visible iris diameter (HVID), along with diagnostic lenses. Slit lamp observation typically offers a preliminary understanding of the anterior eye‘s shape and sagittal height. The clinic‘s fitting set comprises 200 trial scleral lenses with a broad range of parameters. However, the fitting set did not contain a flatter lens than base curve of 8.20 mm and sagittal height (SAG) of 3880 μm at a 15.50 mm chord in the low SAG range.
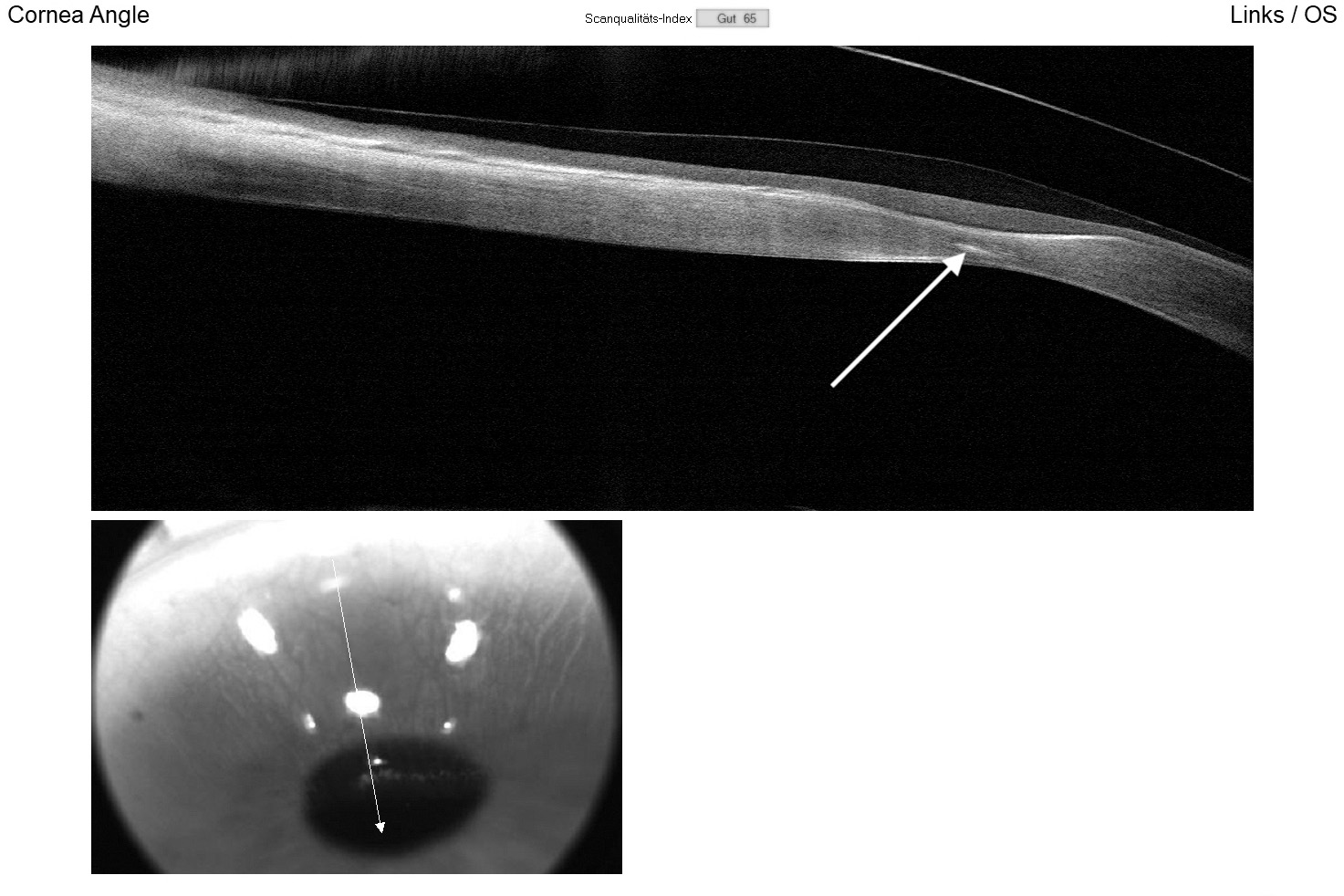
For both eyes, the aforementioned diagnostic lens was applied with sodium fluorescein dye to assess the static fit, including central and peripheral clearance patterns and scleral alignment. Additionally, the vault map obtained through Anterior Segment Optical Coherence Tomography (AS-OCT) was utilised to measure the central lens vault over the cornea, recording 700 μm in the right eye and 150 μm in the left eye. OCT B-scans were conducted in both the flat and steep meridians to evaluate corneo-scleral alignment (Figure 4). Over-refraction was performed to determine the final lens power, resulting in an initial visual acuity (VA) of 20/25 achieved in both eyes.
Prescription scleral lenses were subsequently ordered from Falco Linsen AG, Switzerland, adopting an SMT lens design (scleral and limbal toric design) with reverse geometry for the left eye in Optimum Infinite clear (Contamac).
For the right lens, specifications included a base curve of 8.70 mm, scleral radii of 13.00 mm in the flat and 12.50 mm in the steep meridian, a landing zone diameter of 12.50 mm (Øskl), an overall diameter of 15.60 mm, and a spherical power of −0.62 D. The SAG of the steep meridian at a cord of 12.80 mm measured 2630 μm, while at 15.60 mm, it was 3520 μm. The left lens featured a base curve of 8.50 mm, scleral radii of 12.50 mm in the flat and 12.00 mm in the steep meridian, Øskl of 12.40 mm, an overall diameter of 15.70 mm, and a spherical power of −3.75 D. The SAG of the steep meridian at a cord of 12.80 mm was 3170 μm, increasing to 3910 μm at 15.70 mm.
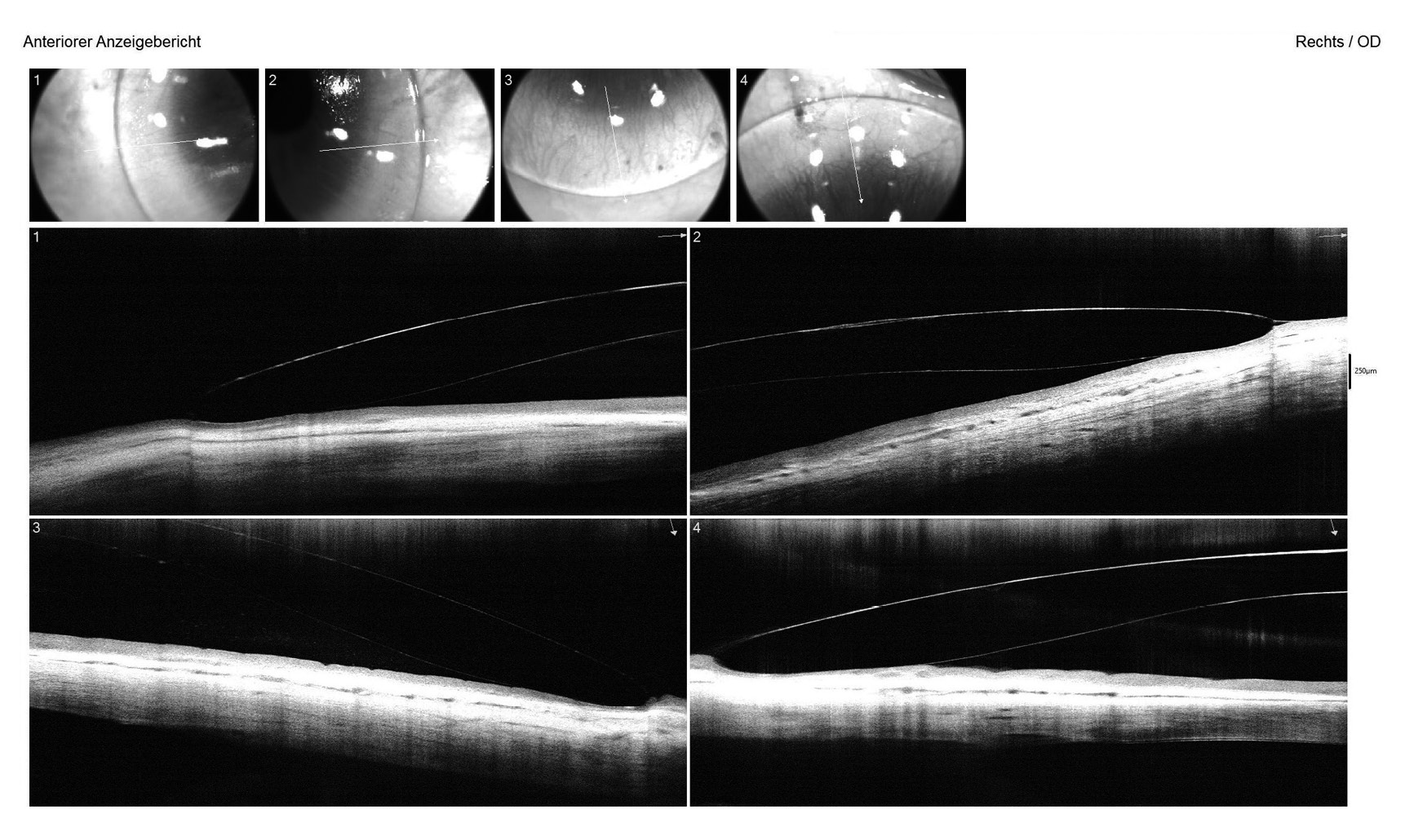
Anschließend wurden die Rezeptlinsen bei der Firma Falco Linsen AG, Schweiz, bestellt; es handelte sich um SMT-Linsen (die ein limbal- und skleraltorisches Rückflächendesign aufweisen) mit einer reversen Rückflächengeometrie für das linke Auge aus dem Linsenmaterial Optimum Infinite Clear (Firma Contamac Ltd., England). Die SMT-Linse für das rechte Auge hatte folgende Werte: Basiskurve 8,70 mm; Radius der Skleralzone 13,00 mm im flachen Meridian und 12,50 mm im steilen Meridian; Durchmesser der skleralen Landezone 12,50 mm (Øskl); Gesamtdurchmesser 15,60 mm; Sphäre −0,62 dpt. Die Scheitelhöhe des steilen Meridians betrug 2630 μm bei einer Sehnenlänge von 12,80 mm beziehungsweise 3520 μm bei einer Sehnenlänge von 15,60 mm. Die SMT-Linse für das linke Auge hatte folgende Parameter: Basiskurve 8,50 mm; Radius der Skleralzone von 12,50 mm im flachen Meridian und 12,00 mm im steilen Meridian; Durchmesser der skleralen Landezone 12,40 mm (Øskl); Gesamtdurchmesser 15,70 mm; Sphäre −3,75 dpt. Die Scheitelhöhe des steilen Meridians betrug 3170 μm bei einer Sehnenlänge von 12,80 mm und 3910 μm bei einer Sehnenlänge von 15,70 mm.
Results
At the dispensing appointment, the lenses were inserted, and the patient was delighted with the immediate improvement in vision. Visual acuity of 20/20 OD and 20/25 OS was achieved. Thorough guidance and instruction on lens handling and care was provided, and follow-up appointments were scheduled at one week, one month, and two months to ensure optimal lens tolerance, visual acuity, and appropriate care. During the two-month follow-up, the patient reported wearing the lenses for 14 hours daily, seven days a week, expressing excitement about the regained vision and the newfound freedom to play soccer without the burden of heavy glasses. The final BCVA with the same lenses increased to 20/16 and 20/20. Objective findings revealed a slight conjunctival hyperaemia and limbal redness, as assessed by the BHVI grading scale after 9 hours of lens wear. Both lenses were slightly decentred downward, as anticipated, with the right lens exhibiting more decentration due to the flat corneal conditions (Figure 5).
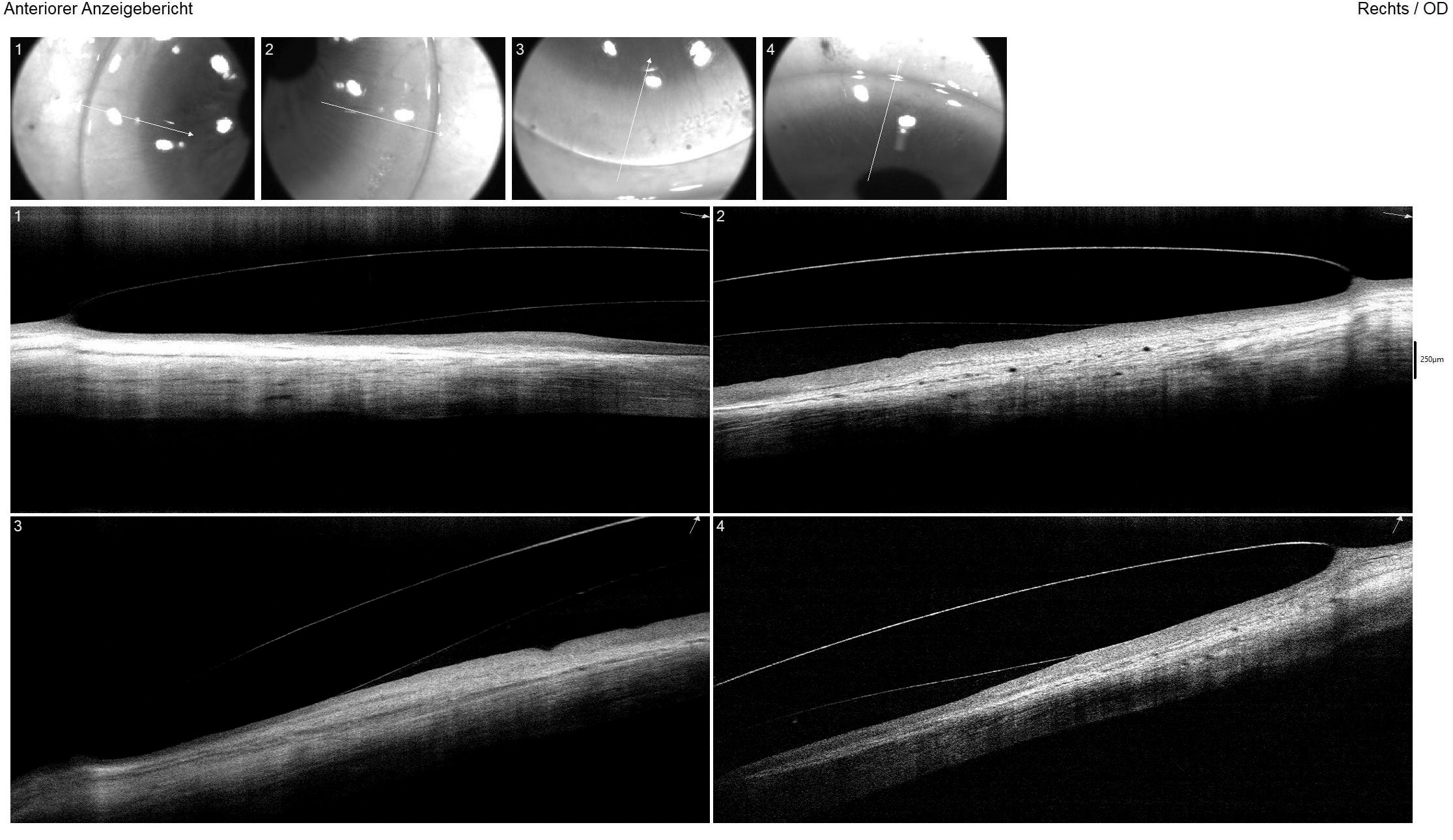
The right lens demonstrated a good fit with slightly above-average central and limbal clearance. Scleral alignment on AS-OCT was regular in both the flat and steep meridians (Figure 6), with no impingement or blanching observed in slit lamp examination. The left lens had minimal central clearance, and a slight contact was noted superiorly at the protrusion but showed no positive fluorescein staining and was thus well-tolerated. Follow-up appointments at six and twelve months, along with lens replacement at twelve months, were scheduled.
During the six- and twelve-month follow-up examinations, compliance, vision, and physiological tolerance of the scleral lenses were confirmed. The lenses were comfortably worn for 15 hours a day with excellent vision, and visual acuity remained unchanged at 20/16 in the right eye and 20/20 in the left eye. Slit lamp examination showed no positive staining, neovascularisation, or increased redness.
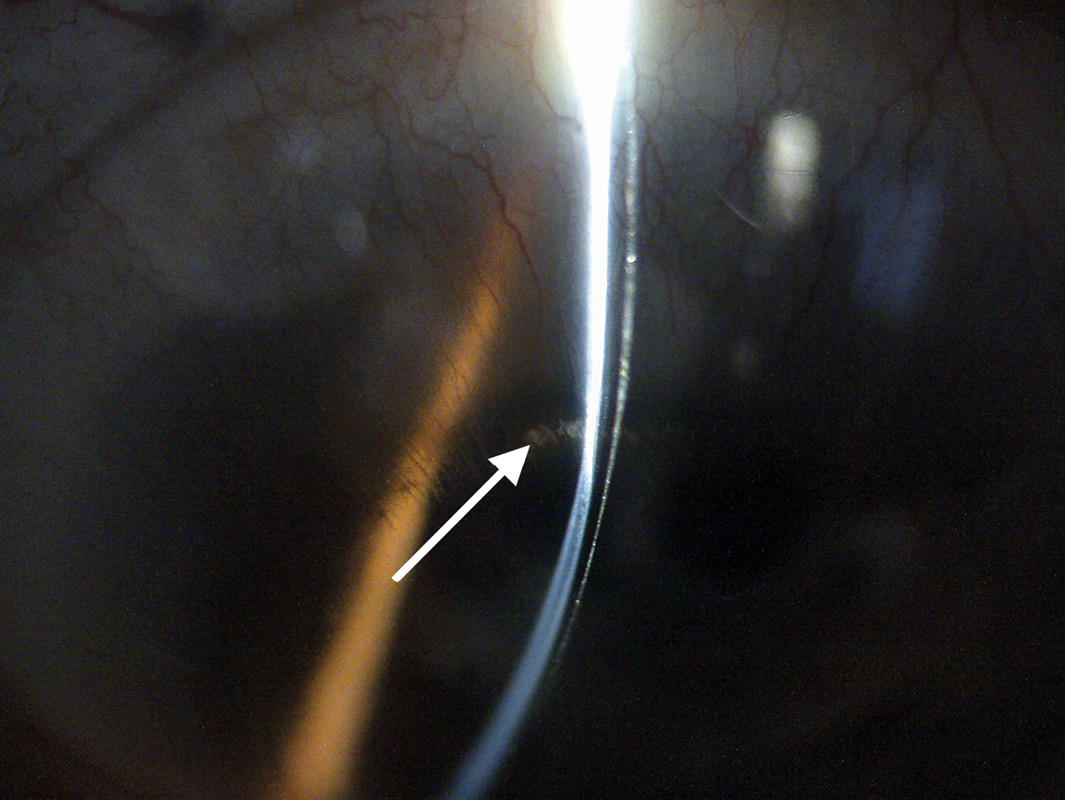
On the left eye, near the superior limbus, minor lipid leakage in the area where the stroma was thinnest was noted (Figure 7). This observation, previously identified by the cornea specialist at the six-month examination after CXL, was deemed clinically not relevant. The cornea specialist also noted that topography, pachymetry, and limbal neovascularisation remained unchanged in both eyes.
During the six- and twelve-month follow-up examinations, compliance, vision, and physiological tolerance of the scleral lenses were confirmed. The lenses were comfortably worn for 15 hours a day with excellent vision, and visual acuity remained unchanged at 20/16 in the right eye and 20/20 in the left eye.
Slit lamp examination showed no positive staining, neovascularisation, or increased redness. On the left eye, near the superior limbus, minor lipid leakage in the area where the stroma was thinnest was noted (Figure 7). This observation, previously identified by the cornea specialist at the six-month examination after CXL, was deemed clinically not relevant. The cornea specialist also noted that topography, pachymetry, and limbal neovascularisation remained unchanged in both eyes.
Discussion
Keratoglobus, a subtype of corneal ectasias, is an additional indication for scleral lenses. It is a rare noninflammatory disease characterised by global thinning from limbus to limbus and corneal protrusion.8,9 Primarily considered congenital and bilateral, keratoglobus is also associated with connective tissue disorders such as Ehlers-Danlos disease type VI and Marfan syndrome. Additionally, it can be acquired through chronic marginal blepharitis and vernal keratoconjunctivitis.9
In this specific case, the patient exhibited very low corneal refractive power and a below-average horizontal visible iris diameter (HVID). This presentation prompted the need to differentiate it from cornea plana. Notably, in 78 cases of autosomal recessive cornea plana in Finland, an average corneal refractive power of 30.61 D, ranging from 18.5 to 44.0 D, was identified. The shallow anterior chamber and small average HVID of OD 9.79 and OS 9.86, ranging from 8.00 to 11.00 mm and 7.00 to 13.00 mm, respectively, were also characteristic.10 However, the patient under consideration demonstrated a larger HVID. Additionally, AS-OCT and slit lamp examinations revealed a normal anterior chamber angle, contrasting with cornea plana cases at risk for angle closure glaucoma due to a very narrow anterior chamber.11 Therefore, the diagnosis of cornea plana applied only partially, and further differentiation from posterior amorphous corneal dystrophy was established by the global thinning of the cornea. A documented case involving both keratoglobus and polymorphic corneal dystrophy in the literature 12 validated he ophthalmologist‘s assessment of both diseases.
Flat corneal properties pose challenges in fitting rigid contact lenses (RCL) for achieving a centred fit.13–15 For instance, patients encounter similar challenges in lens fitting post radial keratotomy (RK). A case report has indicated that scleral lenses offer a favourable option for a more centred and stable fit due to corneal vaulting compared to RCLs.16 This perspective aligns with the author‘s experience, suggesting that good centration can be achieved by default with scleral lenses in patients with very flat and irregular corneas, such as those post-RK, LASIK, or with cornea plana, owing to their corneal vaulting and larger diameter compared to RCLs.
Despite the challenges, achieving acceptable centration of scleral lenses can be difficult in cases like this one. Even in normal eyes, scleral lenses can have a tendency to decentre infero-temporally due to factors such as gravity, upper eyelid forces, and irregular anterior scleral shape.17–19 On a flat ocular surface, where higher positive refractive power is typically required, this challenge is exacerbated, as it increases the thickness and weight of the lens, making decentration more likely.17 Strategies to address this issue include selecting an optimal landing zone diameter and increasing the overall diameter of the lens to enhance centration. However, in this case, achieving adequate vaulting in the area of the superonasal protrusion of the left eye was challenging, requiring a gentle touch that had to be tolerated and monitored.
Diagnostic fitting of scleral lenses assisted by Anterior Segment Optical Coherence Tomography (AS-OCT) proves helpful in achieving optimal corneal clearance, ensuring an adequate width of the landing zone, and correct alignment of the haptic zone.17 Yet, in cases of very flat corneas, it can be challenging as standard diagnostic sets often lack lenses with very low sagittal height (SAG).
Another advantage of scleral lenses over rigid contact lenses (RCLs) post-corneal crosslinking (CXL) is their ability to prevent mechanical stress on the cornea due to their vaulting effect.20,21 Literature offers guidelines on when to start fitting scleral lenses after CXL, ranging from two weeks to two months.20,22–24 A retrospective study found that, on average, corneal RCLs were worn again 2.53 months after CXL.25 Following an epi-off procedure, it is recommended to wait at least three months before fitting RCLs; however, scleral lenses can be fitted earlier after CXL.24 Criteria for initiating lens wear after surgery include complete re-epithelialisation. Most patients will need to continue using topical steroids, being reminded to apply them at least 15 minutes before lens insertion and to remove the lenses if drops must be applied throughout the day.
Changes in corneal curvature are anticipated after CXL, and this is considered a desired effect of the procedure.21,25,26 The flattening of the corneal curvature can impact the fit of RCLs, necessitating frequent refitting.24 However, scleral lenses, based on literature and the author‘s experience, are minimally affected by corneal curvature changes due to CXL,20,24 making them a favourable choice when prompt visual rehabilitation is required after the procedure. The author often initiates scleral lens fitting prior to a planned CXL treatment, allowing patients, in consultation with the treating ophthalmologist, to begin wearing their lenses as early as about three weeks after the procedure.
Conclusion
This case exemplifies the advantages of scleral lenses and their remarkable visual outcomes in the context of an atypically flat keratoglobus. Following corneal crosslinking, scleral lenses offer a shorter time for wearing resumption compared to rigid contact lenses due to their ability to vault over the cornea. In instances of central and peripheral corneal irreguarities, as well as extremely flat or other complex corneal conditions, scleral lenses prove to be a reliable approach for visual rehabilitation, surpassing the optical and geometric limitations of other contact lens types. While lens centring can still present a challenge, it is generally easier to achieve with scleral lenses than with rigid contact lenses. Careful and considered diagnostic fitting, involving the selection of an appropriate lens design and aided by Anterior Segment Optical Coherence Tomography, coupled with regular follow-up examinations, facilitates long-term, physiologically well-tolerated wear of scleral lenses. This significantly enhances the daily lives of individuals who would otherwise face visual impairment challenges.
Conflict of interest
The author has no conflict of interest with regard to the methods, products and devices mentioned in the article.
